Abstract
Objective
Survivin is a member of the inhibitor of apoptosis family. Our previous study showed that survivin expression could be strongly induced by long-term, high-fat diet (HFD) exposure in vivo. It could also be induced by insulin through the PI3K/mTOR signaling pathway in vitro. Therefore, we hypothesized that under certain conditions, survivin expression might be required for adipocyte function. In the current study, we aim to further investigate the regulation of survivin expression in mature adipocytes upon various nutritional stimuli and the role of survivin using adipocyte-specific survivin knockout (SKO) mice.
Methods
SKO mice were obtained by crossing survivinflox/flox mice with Adiponectin-Cre+/- mice. The overall metabolic phenotype was observed under chow diet (CD) and HFD feeding conditions. The thermogenic program of mice was detected upon cold exposure. The inguinal white adipose tissue (iWAT) and brown adipose tissue (BAT) stromal vascular fraction cells were isolated and differentiated into mature adipocytes, and the effects of survivin deletion on mature adipocyte function were detected in vitro.
Results
Survivin expression in adipose tissue and adipocytes was regulated by short-term nutritional stress both in vivo and in vitro. The postnatal development of BAT was impaired in SKO mice, which resulted in drastically reduced BAT mass and decreased expression of the thermogenic protein Ucp1 in 24-week-old mice fed with CD. After HFD feeding, the iWAT and BAT mass of SKO mice were significantly decreased, causing ectopic lipid accumulation in the liver, which was associated with insulin resistance and glucose intolerance. Upon cold exposure, the expression of thermogenic genes and proteins was markedly reduced in BAT and iWAT of SKO mice, accompanied by abnormal mitochondrial structure and induced autophagy. Consistently, thermogenic program and mitochondrial oxidative phosphorylation were reduced in survivin-depleted brown and beige adipocytes in vitro.
Conclusions
Our findings showed that survivin could be regulated by nutritional stress in adipocytes and revealed a new role of survivin in maintaining normal BAT mass and positively regulating the thermogenic program and mitochondrial oxidative phosphorylation.
Keywords: Survivin, Obesity, Brown adipose tissue development, Thermogenesis, Autophagy
Highlights
-
•
Survivin expression in adipocytes is regulated by nutritional stress.
-
•
Survivin is required for maintaining BAT mass and thermogenic program in mice.
-
•
Survivin deletion in adipocytes impairs glucose homeostasis when exposed to HFD.
-
•
Survivin is required for activation of thermogenic program response to cold exposure.
-
•
Adipocyte-specific deletion of survivin induces autophagy in vivo and in vitro.
1. Introduction
Adipose tissue is highly dynamic tissue and is readily remodeled upon exposure to various nutritional stresses [1]. Among the many cell types in adipose tissue, adipocytes are specialized cells that store energy in the form of triacylglycerols and mobilize energy as fatty acids, coordinating the feeding and fasting cycle [2]. In the setting of nutrient excess, adipose tissue expands to store extra energy by hypertrophy or hyperplasia, and the ability of adipocytes to effectively sequester lipid intracellularly prevents ectopic lipid accumulation in other tissues such as muscle, liver, and heart tissue, preventing metabolic dysregulation and progressive insulin resistance [3]. Mature adipocytes express nutrient-sensing proteins, which regulate adipose tissue remodeling upon over-nutrition [4]. Thus, further study of the specific function of this group of nutrient-sensing proteins in adipocytes may provide new insights into adipose tissue physiology and healthy fat expansion in obesity.
Adipose tissue is a complex metabolic organ composed of many cell types, including mature adipocytes, preadipocytes, fibroblasts, immune cells (macrophages, neutrophils, lymphocytes), and endothelial cells [[5], [6], [7]]. Adipocytes have been divided into three major classes. White adipocytes contain a large unilocular lipid droplet and a small number of mitochondria. In contrast, brown adipocytes contain small multilocular lipid droplets and higher amounts of mitochondria; they are specialized in dissipating stored chemical energy in the form of heat through the action of uncoupling protein 1 (Ucp1) [5]. Beige adipocytes are the brown-like cells located within WAT [8]. Thermogenesis in brown and beige adipocytes is mainly regulated by β-adrenergic signaling and cAMP levels [9]. In addition to sympathetic tone, cellular energy sensing is also the driving force that regulates the transcriptional network of browning [10]. Mammalian/Mechanistic target of rapamycin (mTOR) is known as a critical energy sensor; however, recent studies have indicated that mTOR also regulates non-shivering thermogenesis and the development of brown/beige adipocytes [11]. Diet-induced thermogenesis (DIT), which is induced by meal intake, is another way of regulating non-shivering thermogenesis [12]. It has been reported that peri-prandial secretion of gut hormones, like cholecystokinin and glucagon-like peptide 1, activates DIT via stimulating the central efferent tone of sympathetic innervation in BAT [13]. Another gut hormone, secretin, which is induced during meal intake, could directly activate DIT in BAT by stimulating lipolysis upon binding to secretin receptors in brown adipocytes [14]. Brown/beige adipocytes maintain systemic metabolic homeostasis by dissipating energy and acting as metabolic sinks for various substrates, such as glucose, lipids, and many other metabolites, which is different from the role of white adipocytes in nutrient handling. Whether the nutrient-sensing proteins in brown/beige adipocytes directly modulate thermogenic function has not been extensively studied.
Survivin, which is encoded by the birc5 gene, is a member of the inhibitor of apoptosis family of proteins and functions to inhibit apoptosis and promote cell division. Survivin is highly expressed in most common malignancies and plays an important role in cancer development through preventing apoptosis and regulating the cell cycle [15]. Further studies have revealed that survivin expression is developmentally regulated in normal tissue, with low expression in most terminally differentiated adult tissues [16]. However, accumulating evidence shows that survivin is expressed in normal tissue and terminally differentiated mature cells and that its expression is significantly induced under certain conditions [[17], [18], [19]]; this suggests that survivin expression is not cancer-specific and that it may also play an important role in terminally differentiated cells. To our knowledge, studies on survivin expression and function in adipose tissue are scarce. Ejarque et al. reported that survivin protein expression in human adipocyte-derived stem cells (hASCs) isolated from obese individuals is significantly elevated, which protects hASCs from apoptosis [20]. Another study reported that survivin expression in visceral adipose tissue is higher in obese animal models than in lean controls, and it decreases after weight loss via energy restriction [21]. Our previous work has revealed that survivin expression in mature adipocytes from WAT could be highly induced by long-term HFD feeding in vivo [22]. In vitro, insulin robustly increases survivin expression through activating the PI3K/mTORC1 signaling pathway in adipocytes. We further verified that induced survivin expression inhibits isoproterenol (ISO)-stimulated and TNFα-induced lipolysis in adipocytes, suggesting that survivin may facilitate adipocyte maintenance in response to inflammatory conditions. In the present study, we used adipocyte-specific SKO mice to investigate the role of survivin in adipose tissue homeostasis and energy metabolism.
Here we show that survivin expression in adipose tissue and adipocytes is upregulated upon excessive nutrition in vivo and in vitro, and after withdrawing certain nutritious content from the cell culture medium, survivin is rapidly degraded. More importantly, we found that adipocyte-specific survivin knockout leads to defects in normal BAT mass maintenance and thermogenic program in vivo. Moreover, survivin deficiency in adipocytes not only reduces BAT mass and decreases Ucp1 levels upon HFD feeding but also leads to impaired glucose tolerance and insulin sensitivity. We further show that the activation of BAT and browning of inguinal white adipose tissue (iWAT) are inhibited in SKO mice upon cold exposure, which is accompanied by induced autophagy activity. Overall, our study shows that survivin is critical for the maintenance of BAT mass and thermogenic program in adipocytes, particularly under conditions of metabolic stress.
2. Materials and methods
2.1. Mice and animal experiments
Animal experiments were approved by the ethics committee of Shanghai Jiao Tong University Affiliated Sixth People's Hospital and were carried out in strict accordance with the Guidelines for the Care and Use of Laboratory Animals of Shanghai Jiao Tong University Affiliated Sixth People's Hospital. Mice were maintained in a pathogen-free environment with constant temperature (22 ± 2 °C) and relative humidity (50 ± 10%) under a 12/12-h light/dark cycle. Mice were fed CD with free access to water. For diet-induced obesity studies, mice were fed HFD (60% fat, Research Diets, D12492). Survivinflox/flox mice were obtained from Dr. Lijian Hui's lab with the permission of Professor Edward Conway. The schematic diagram of the birc5 gene locus and the loxP sites is shown in Figure S1A. SKO mice were generated by crossing survivinflox/flox mice with Adiponectin-Cre+/- mice, which were a kind gift from Dr. Junli Liu from our institute. The adiponectin-Cre+/- mice were also used and validated by another recent study [23]. After the Cre/loxp excision, the exon1–4 of Birc5 gene was deleted. Genotyping was performed by PCR of tail or toenail DNA as described previously [24]. At the end of all animal experiments, mice were anesthetized with isoflurane and euthanized by cervical dislocation. Tissues were collected, rapidly frozen in liquid nitrogen, and stored at −80 °C until use. For fasting and refeeding experiments, 8-week-old C57BL/6 mice were randomly divided into two groups, and both groups fasted for 12 h (overnight). Then all mice were intraperitoneally injected with 15 mg/kg MG132. Mice in the refed group were fed with CD for 4 h, while mice in the fast group fasted for an additional 4 h before tissue collection. For cold exposure experiments, 9-week-old male Con and SKO mice were housed in a single cage with free access to water and food. The housing temperature was decreased gradually (16 °C for the first 24 h, 10 °C for another 24 h, and 4 °C for the final 24 h). Eight-week-old C57BL/6 mice were subcutaneously implanted with mini-osmotic pumps (Alzet 2001) perfusing 1.0 ml/h of CL-316,243 (1 mg/kg, Sigma, C5976) for 1 week to induce BAT activation and WAT browning. After euthanasia, adipose tissues were harvested, and the stromal vascular fraction (SVF) and the mature adipocyte fraction were separated as previously described [22]. Briefly, adipose tissues were minced thoroughly and digested in filtered DMEM containing 1.5% BSA and 0.2% collagenase (Sigma, C6885) for 30–60 min at 37 °C with shaking. Digested tissues were carefully filtered through 70-μm cell strainers and centrifuged for 10 min at 2400 rpm to pellet the stromal vascular cells. The floating mature adipocyte fraction layer was collected for further use.
2.2. Glucose tolerance test and insulin tolerance test
For the intraperitoneal glucose tolerance test (IPGTT), mice were intraperitoneally injected with glucose (1 g/kg body weight) after overnight fasting. For the insulin tolerance test (ITT), mice were fasted for 6 h and injected with insulin (Lily, HI0240) at a concentration of 1 U/kg for CD mice and 1.5 U/kg for HFD mice. Blood glucose was measured using tail vein blood with a glucometer (Roche, Switzerland) at 0, 15, 30, 60, and 120 min after the injection.
2.3. Histology
Tissues were fixed in 4% (v/v) formaldehyde in PBS for 24 h at 4 °C and embedded in paraffin for histological analysis. Adipose and liver tissues were stained with hematoxylin and eosin (HE) following standard protocols. For immunohistochemistry, the sections were blocked with goat serum, to reduce non-specific staining, and incubated with rabbit anti-Ucp1 (Abcam, ab10983, diluted 1:200), followed by incubation with HRP-conjugated goat anti-rabbit (PV-6001, ZSGB-BIO, Beijing, China).
2.4. Transmission electron microscopy
Small fragments of BAT were obtained from Con and SKO mice; the fragments were cut into pieces of about 1 mm3 by using a sharp blade, and the pieces were then placed in Petri dishes. Tissue fragments were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) and stored at −4 °C for 3 h. Specimens were then washed using 0.1 M phosphate buffer (pH 7.4), postfixed in 1% OsO4 (pH 7.4) for 2 h at room temperature, dehydrated in ethanol, and embedded in an Acetone–EMBed 812 mixture. Thin sections were obtained using an ultra-microtome (Leica, Germany), stained with 2.6% lead citrate, and examined with a transmission electron microscope (HITACHI, Japan). Mitochondrial density was analyzed using Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA). Briefly, 3 complete and clear images with 1500 × 10 μm scale were selected for each sample, then the number of mitochondria in 5 equal sections from each image was counted manually.
2.5. Transcriptome analysis for BAT
RNA was extracted from BAT with the RNAsimple Total RNA Kit (50 Tiangen #DP419) according to the manufacturer's protocol. Libraries were prepared using the TruSeq®
RNA Sample Preparation Kit (Illumina, RS-122-2001). Library construction and Illumina sequencing were performed at Sinotech Genomics. Paired-end sequence files were mapped to the reference genome (GRCm38.91) using Hisat2 (version 2.0.5). R package edgeR was used to perform Differential expression analysis. Differentially expressed genes (DEGs) were identified according to the following criteria: |fold change| > 1.5 and false discovery rate <0.05.
2.6. Cells
3T3-L1 cells were purchased from the American Cell Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in complete culture medium containing DMEM (Gibco, 11,995, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, 10,099–141, Australia) and 1% penicillin/streptomycin (Gibco, #15140–122). After cells reached 100% confluence (Day 0), the culture medium was replaced with induction medium containing 0.5 mM IBMX (Sigma, I7018), 1 μM dexamethasone (Sigma, D4902), and 1 μM insulin (Lily, HI0240). Two days after induction (Day 2), cells were maintained in medium containing 1 μM insulin for another 2 days and then maintained in complete medium.
2.7. SVF isolation and differentiation
SVFs were isolated from the iWAT or BAT from 6 to 8-week-old Con, SKO, or survivinflox/flox male mice as previously described [22]. For the induction of differentiation into brown or beige adipocytes, SVFs were grown to confluence, treated for two days with induction medium (DMEM supplemented with 10% FBS, 0.5 mM IBMX, 1 μM dexamethasone, 1 μM insulin, 50 nM T3 (Sigma, T2877) and 5 μM rosiglitazone (Sigma, R2408)), and subsequently maintained for another 4–5 days in DMEM supplemented with 5 μg/ml insulin, 50 nM T3, and 5 μM rosiglitazone. To analyze autophagic activity in vitro, beige adipocytes were starved in starvation medium (DMEM containing 0.2% BSA) for 2–4 h.
2.8. RNA isolation and real-time PCR analysis
Total RNA was extracted using TRIzol reagent (Invitrogen, 15,596,018) according to the manufacturer's instructions. cDNA was synthesized using a PrimeScript® RT reagent Kit (Takara, RR047B). Quantitative real-time PCR analysis was conducted using SYBR Premix Ex Taq (Vazyme, Q511-03) in a LightCycler480 PCR system (Roche, Germany). Primer sequences are listed in Table S1.
2.9. Western blot analysis
Protein samples were prepared with RIPA lysis buffer (Beyotime, P0013B) supplemented with phosphatase inhibitor cocktail (Roche, 4,906,845,001) and protease inhibitor cocktail (Roche, 04,693,132,001). Western blotting was performed as previously described [22] with the following primary antibodies: anti-survivin (Cell Signaling Technology, 2808 S), anti-p-PS6K (Thr389) (Cell Signaling Technology, 9234), anti-Ucp1 (Abcam, ab10983), anti-Pgc1a (Millipore, AB3242), anti-SQSTM1/P62 (Cell Signaling Technology, 5114 S), anti-β-actin (Cell Signaling Technology 3700), and anti-α-tubulin (Sigma, T6199).
2.10. Lipid assay and lipolysis measurement
Hepatic triglyceride (TG) content was detected using the Biovision Triglycerides Quantification Colorimetric/Fluorometric Kit (#K622-100) following the manufacturer's protocol. Differentiated mature brown and beige adipocytes were treated with 5 μM isoproterenol in serum-free DMEM containing 0.2% BSA for 3 h. The glycerol content in the supernatant was measured using GPO-Trinder reagent (Sigma, FG0100, St. Louis, MO, USA) according to the manufacturer's instructions and used as an index for lipolysis. Results were corrected for cellular protein concentrations.
2.11. Oxygen consumption rate measurements
SVFs were seeded in an XF24 V28 microplate (Seahorse Bioscience) precoated with poly-l-lysine and subsequently induced to differentiate into brown or beige adipocytes. At day 4 of differentiation, the oxygen consumption rate (OCR) was measured as previously described using an XF24 analyzer with the Mito stress kit (Agilent, 103,015) in accordance with the manufacturer's instructions [25]. Briefly, the cells were washed and incubated with prewarmed Seahorse XF base medium with 25 mM glucose, 2 mM sodium pyruvate, and 2 mM glutamine (pH 7.4) in a non-CO2 incubator (Seahorse Bioscience) at 37 °C for 1 h. The drug injection ports of the sensor cartridge were loaded with 75 μl respiratory inhibitors, and all following measurements were conducted for three cycles. After measuring basal OCR, 2 μM oligomycin, 1 μM FCCP, or 1 μM rotenone/1 μM antimycin was added to measure uncoupled respiration, maximal respiration, and non-mitochondrial respiration, respectively. The initial OCR values were automatically calculated by Seahorse XF24 software (Wave, Seahorse Bioscience), and the final OCR results were standardized to total protein content in each well.
2.12. Analysis of autophagic flux
For autophagic flux measurements, iWAT SVFs from Survivinflox/flox mice were differentiated into beige adipocytes, and on day 3 they were infected with adenovirus expressing Cre recombinase for 12 h in maintenance medium to delete Birc5 gene in primary adipocytes. Cells were infected with adenoviruses that do not express Cre-recombinase were used as controls. After 48 h, beige adipocytes were transfected with adenoviruses harboring mRFP-GFPL-C3 (Hanbio Biotechnology, China). The cells were then cultured in serum starvation medium containing 0.2% BSA for 2 h. GFP and RFP puncta were detected using a confocal laser scanning microscope (LSM 880 Carl Zeiss, Germany).
2.13. Statistical analyses
All data are presented as mean ± standard error of the mean (SEM). The two-tailed unpaired Student t-tests or two-way ANOVA with Bonferroni corrections was used to assess the statistical significance. P < 0.05 was considered to indicate statistical significance. GraphPad Prism 9 and Microsoft Excel were used for calculations.
3. Results
3.1. Survivin/Birc5 expression in adipose tissue and adipocytes is regulated by nutritional stress both in vivo and in vitro
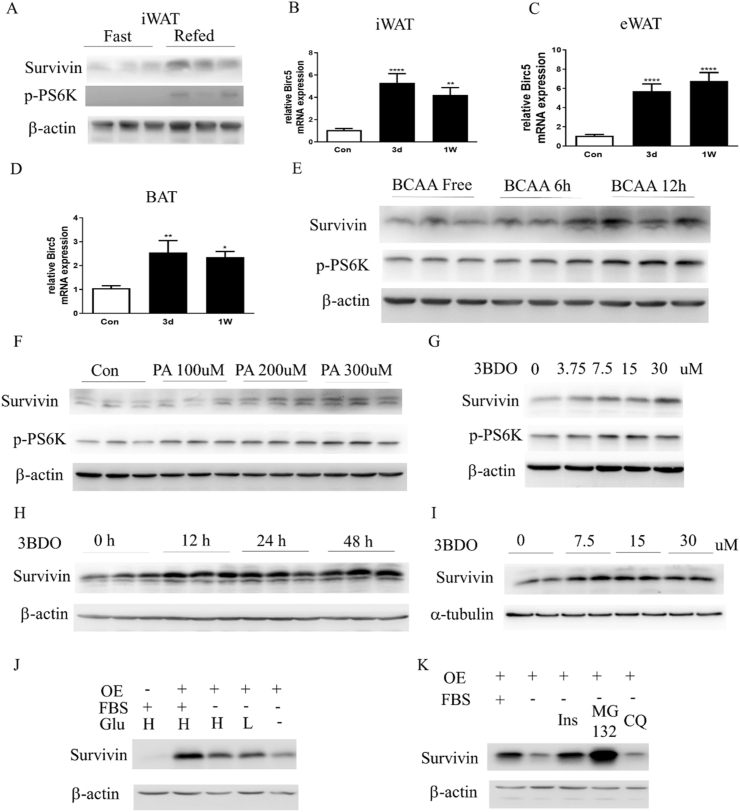
In our previous work, we found that survivin could be significantly upregulated after long-term HFD feeding in mature adipocytes of WAT [22]. In this study, to confirm that survivin expression in adipocytes is sensitive to short-term nutritional stimuli, survivin protein levels were detected in adipose tissue from 8-week-old mice under fasting (16 h) or refeeding (4 h) conditions. As shown in Figure 1A, survivin protein levels in iWAT significantly increased after refeeding. The levels of p-PS6K, which is a signal transducer downstream of the nutrient sensor mTORC1, were significantly higher in iWAT from refed mice. Next, we investigated the effects of short-term HFD feeding on birc5 gene expression in adipose tissue. Compared to control-mice fed with CD, HFD feeding for 3 days was sufficient to induce the transcriptional expression of birc5 gene in iWAT, epididymal WAT (eWAT), and BAT (Figure 1B–D).
Figure 1.
Survivin expression in adipose tissue and adipocytes is regulated by nutritional stress both in vivo and in vitro. (A) Survivin expression in iWAT of 8-week-old mice under fasting (16 h) or refeeding (4 h) conditions was determined by western blot. (B–D) Birc5 gene expression in the iWAT (B), eWAT (C), and BAT (D) of 14-week-old mice fed with normal CD or HFD for 3 days or 1 week, as determined by qRT-PCR. (E) Survivin expression was detected by western blot in 3T3-L1 adipocytes, which were treated with branched chain amino acid (BCAA) for the indicated times. (F) Survivin expression in 3T3-L1 adipocytes treated with phosphatidic acid (PA) at the indicated concentrations for 12 h. (G, H) Survivin expression in 3T3-L1 adipocytes treated with 3-benzyl-5-((2-nitrophenoxy) methyl)–dihydrofuran-2 (3 h)-one (3BDO, an inhibitor of mTORC1) at the indicated concentrations for 24 h (G) or with 30 μM 3BDO for the indicated times (H). (I) Survivin expression in differentiated beige adipocytes treated with 3BDO at the indicated concentrations for 24 h. (J) Protein levels of survivin in 3T3-L1 adipocytes overexpressing survivin after withdrawing FBS or/and glucose for 3 h, as detected by western blot. (K) Survivin was detected in 3T3-L1 adipocytes overexpressing survivin under FBS-free conditions in the presence of 100 nM insulin, 10 μM MG132, and 60 μM CQ. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
We previously revealed that survivin expression in adipocytes is induced by insulin treatment via activating the mTORC1 pathway in vitro [22]. To confirm that survivin is regulated by the nutritional status in adipocytes, survivin expression was detected in 3T3-L1 adipocytes cultured in branched chain amino acid (BCAA) free and BCAA-containing medium. BCAA significantly increased the p-PS6K content, and survivin protein levels also increased in the presence of BCAA in a time-dependent manner (Figure 1E). It was previously reported that phosphatidic acid (PA) could activate the mTORC1 pathway [26]. Our results showed that PA dose-dependently induced survivin protein expression, with strong induction observed at 300 μM (Figure 1F). According to a previous study, 3-benzyl-5-((2-nitrophenoxy)methyl)–dihydrofuran-2 (3 h)-one (3BDO), a small molecule, could activate the mTOR pathway by targeting FK506-binding protein 1 A [27]. We tested whether 3BDO could regulate survivin expression while activating mTORC1. After 3BDO treatment, survivin expression increased in a dose- and time-dependent manner in adipocytes (Figure 1G,H). Consistently, survivin expression was also upregulated by 3BDO in differentiated beige adipocytes (Figure 1I). These results suggest that survivin expression in adipocytes is regulated by the nutrition-sensing mTORC1 pathway. Next, we tested the degradation of overexpressed survivin in adipocytes under nutrient deprivation conditions. Compared to complete culture medium (DMEM containing 10% FBS and 4.5 g/L glucose), survivin expression decreased significantly in FBS-free medium containing 0.2% BSA (Figure 1J). In addition, survivin protein levels decreased more drastically in the absence of both FBS and glucose. One previous study reported that survivin was degraded via the ubiquitin–proteasome pathway in a cell cycle-dependent manner in HEK 293 cells [28]. However, another study showed that BafA (an autophagy inhibitor) and MG132 (a proteasome inhibitor) both inhibited survivin degradation during glycolysis inhibition in neuroblastoma cell lines, indicating that survivin could be degraded through both proteasomal and autophagy–lysosomal pathways [29]. Our results showed that MG132 can block survivin degradation after withdrawing FBS, and CQ was unable to inhibit survivin degradation (Figure 1K), suggesting that in adipocytes under nutrition deprivation conditions, survivin is mainly degraded via the proteasomal pathway. Moreover, survivin degradation was rescued upon treatment with insulin in FBS-free medium.
3.2. Survivin is required for maintaining BAT mass and thermogenic function in mice
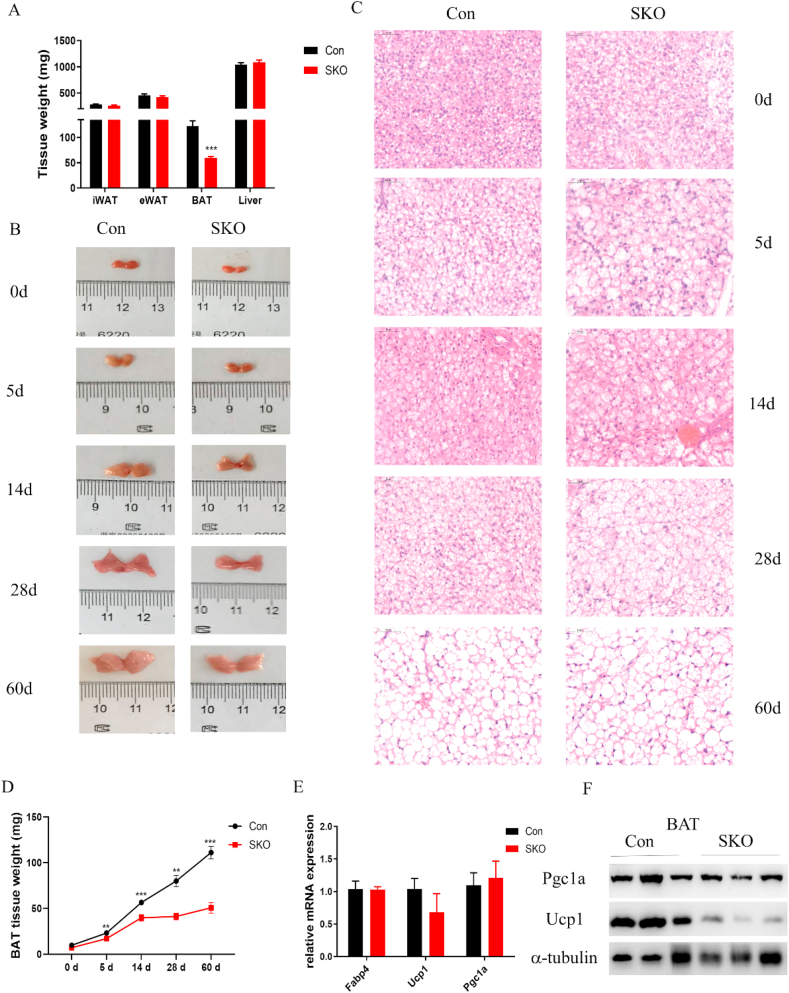
To investigate the function of survivin in adipocytes, we generated adipocyte-specific SKO mice (referred to as SKO mice) by crossing survivinflox/flox mice with Adiponectin -Cre transgenic mice. Reproductive function and female nursing were not affected by survivin deletion in adipocytes, and all pups were viable and born at the expected Mendelian ratio. PCR using three primers was conducted to analyze for the presence of the survivin flox allele (a 577-bp product) or the deleted allele (a 420-bp product). As showed in Figure S1B, Cre recombination of the survivin locus generated a deletion product in iWAT, eWAT, and BAT from SKO mice, which was absent in all tissues from survivinflox/flox controls and in gastrocnemius muscle, and soleus muscle from SKO mice. Western blot results also showed that survivin protein levels were significantly decreased in iWAT and BAT (Figure S1C and D). There was no difference in body weight of 24-week-old SKO and Con mice fed CD (Figure S2A), while the BAT tissue mass was significantly reduced in SKO mice; iWAT, eWAT, and liver tissue weight of SKO mice were not altered (Figure 2A). Therefore, we measured the BAT tissue weight of Con and SKO mice at different time points after birth to determine whether the difference in BAT mass was prenatal or postnatal. The gross appearance and mass of BAT in the two groups were similar at 0 days after birth. The weight of BAT in Con mice continuously increased until 60 days after birth; however, the weight of BAT in SKO mice increased at a slower rate, especially after 14 days of age (Figure 2B,D). HE staining revealed no significant difference in BAT morphology between the two groups at 0 days after birth, and as the mice aged, the lipid droplets became slightly larger in BAT of SKO mice (Figure 2C). We further investigated the thermogenic program of BAT in 24-week-old SKO mice fed CD. Expression of the key thermogenic protein Ucp1 decreased in BAT of SKO mice, although no significant difference in transcriptional levels of the differentiational marker gene Fabp4, ucp1, and pgc1a was observed (Figure 2E,F). These data indicated that survivin plays an important role in maintaining normal BAT development and thermogenic program.
Figure 2.
Survivin is required for maintaining BAT mass and thermogenic program in mice. (A) The major metabolic tissue weight of 24-week-old Con and SKO mice fed CD (n = 7–9 for each group). (B, C) Representative gross appearance (B) and HE staining (C) of BATs from Con and SKO mice at 0, 5, 14, 28, and 60 days after birth. (D) BAT tissue weight of Con and SKO mice at 0, 5, 14, 28, and 60 days after birth (n = 5–8 for each group). (E) Differentiational and thermogenic marker genes expression in BAT from 24-week-old Con and SKO mice fed CD (n = 4–6 for each group). (F) Expression of the Pgc1a and Ucp1 in BAT from 24-week-old Con and SKO mice fed CD. Significance was determined by the unpaired two-tailed Student t-test. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
3.3. Adipocyte-specific deletion of survivin impairs glucose homeostasis upon HFD exposure
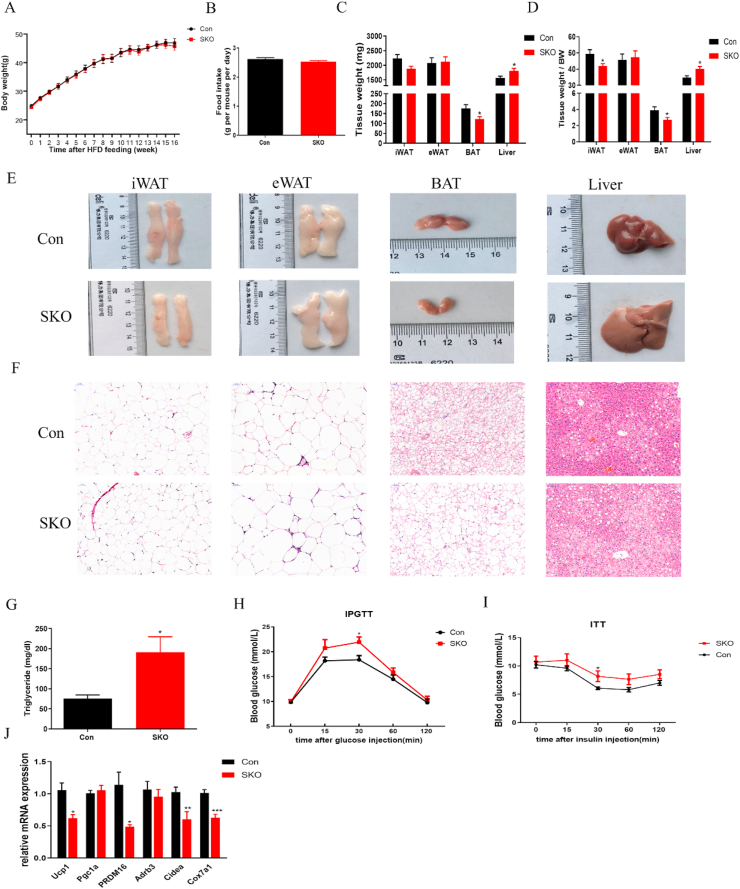
We further investigated the role of survivin in adipose tissue under HFD-induced obesity. After 16 weeks of HFD feeding, the SKO mice had body weights and food intakes similar to Con mice (Figure 3A,B). The BAT weight was still reduced in SKO mice after HFD feeding, accompanied by increased liver weight, and there was no change in iWAT and eWAT weight between the two groups (Figure 3C). In addition, the relative weight of iWAT and BAT to body weight was significantly lower, and the relative weight of the liver was much higher in SKO mice (Figure 3D). Consistently, the iWAT and BAT were smaller, and the liver was larger in SKO mice compared with Con mice (Figure 3E). Furthermore, HE staining showed that adipocytes were slightly smaller in iWAT and visibly larger in BAT of SKO mice, and ectopic lipid accumulation was much more severe in the livers of SKO mice (Figure 3F). The liver TG contents were significantly higher in SKO mice (Figure 3G). To determine the effects of survivin depletion in mature adipocytes on glucose homeostasis, we conducted the GTT and ITT on SKO and Con mice after 12 and 14 weeks of HFD feeding, respectively. SKO mice were more glucose-intolerant than Con mice after 12 weeks of HFD (Figure 3H), and the difference between the two genotypes reached statistical significance at 30 min after glucose injection, while no difference was observed in mice fed CD (Figure S2F). The systemic insulin sensitivity was also impaired in SKO mice fed HFD for 14 weeks (Figure 3I); again, a significant difference was observed at 30 min after insulin injection. Moreover, qPCR analyses showed that several thermogenic marker genes, like ucp1, PRDM16, adrb3, and cidea, were significantly downregulated in BAT from SKO mice under HFD conditions (Figure 3J). These data suggested that survivin depletion in adipocytes impaired systematic glucose tolerance and the insulin response without affecting overall body weight, which might be mediated through inadequate iWAT expansion, impaired BAT thermogenesis, and ectopic lipid accumulation in the liver.
Figure 3.
Adipocyte-specific knockout of survivin impaired the glucose homeostasis when exposed to HFD. (A) The growth curve of SKO and Con mice fed HFD for 16 weeks (n = 7–9 for each group). (B) The food intake during HFD feeding of mice in two groups. (C, D) The tissue weight and the ratio of tissue weight to body weight of Con and SKO mice fed HFD for 16 weeks (n = 6–8 for each group). (E, F) Representative gross appearance and HE staining of iWAT, eWAT, BAT, and liver of Con and SKO mice fed HFD for 16 weeks. (G) The liver triglyceride (TG) contents of Con and SKO mice fed HFD for 16 weeks (n = 6 for each group). (H, I) The intraperitoneal glucose tolerance test (IPGTT) and the insulin tolerance test (ITT) after feeding HFD for 12 weeks and 14 weeks, respectively (n = 6–9 for each group). (J) The thermogenic gene expression of BAT from Con and SKO mice fed HFD for 16 weeks (n = 6–8 for each group). ∗P < 0.05. ∗∗P < 0.01, ∗∗∗P < 0.001.
3.4. Adipocyte-specific deletion of survivin reduces thermogenic activity in response to cold exposure
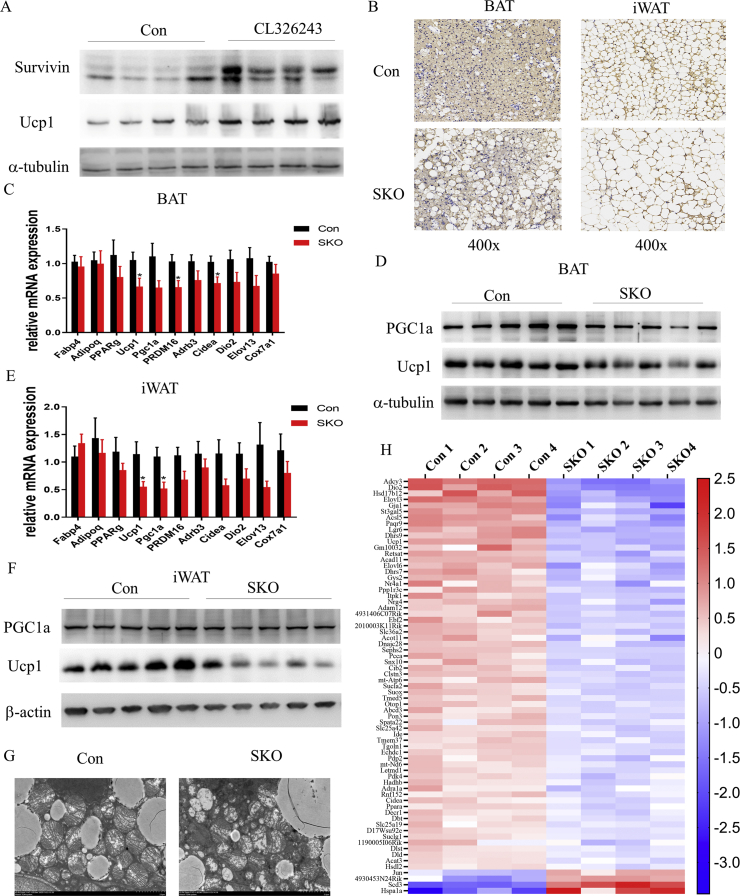
Our experiments with SKO mice indicated that survivin plays a vital role in the thermogenic program of BAT. We next investigated survivin expression in mature adipocytes fraction from BAT after treatment with the β3-adrenergic agonist CL-316,243 for 7 days. Survivin expression was significantly induced by CL-316,243 (Figure 4A), confirming that survivin participates in the regulation of thermogenesis. Next, we tested the thermogenic responses of SKO and Con mice upon cold exposure in vivo. In the initial experiment, four of six SKO mice died on the fourth day of cold exposure; hence, we chose 3-day cold exposure in the following experiments. The BAT mass of SKO mice was still smaller than that of Con mice, and the SKO iWAT was paler (Figure S3D). HE staining showed that both BAT and iWAT of SKO mice contained more adipocytes with larger lipid droplets (Figure S3E), and immunohistochemistry staining further showed that the Ucp1 protein levels were lower in BAT and iWAT of SKO mice (Figure 4B), suggesting impaired thermogenesis in SKO mice upon cold exposure. After 3 days of cold exposure, the expression of most thermogenic genes in SKO mice was reduced in both iWAT and BAT, while the levels of the pan-adipocyte markers fabp4, adipoq, and pparg remained unaffected (Figure 4C,E). Consistently, the Ucp1 and Pgc1a protein levels were significantly reduced in iWAT and BAT of SKO mice in response to 3 days of cold exposure (Figure 4D,F), suggesting that survivin is required for the activation of the thermogenic program in both iWAT and BAT. We further analyzed the mitochondrial ultrastructure by TEM. We found that the mitochondrial density in BAT of SKO mice was reduced after cold exposure (Figure 4G and Figure S3F). Moreover, mitochondria in the BAT of SKO mice presented an aberrant structure, with more swelling and disorganized cristae, although most mitochondria retained a round shape. RNA sequencing analysis of BAT showed that there were 1204 differentially expressed genes (DEGs) between the two genotypes, including 918 upregulated and 286 downregulated genes (Figure S3G). We examined the expression patterns of 254 previously reported BAT-specific genes [30]. Ultimately, 69 significant DEGs were identified, of which 65 were significantly downregulated in SKO BAT (Figure 4H).
Figure 4.
Adipocyte-specific deletion of survivin reduced thermogenic activity in response to cold exposure. (A) The expression of survivin and Ucp1 in mature adipocytes of BAT from Con and CL-316,243-treated mice. (B) Immunohistochemistry staining of iWAT and BAT from Con and SKO mice after 3 days of cold exposure using anti-Ucp1. (C) Expression of pan-adipocyte and thermogenic marker genes in BAT from Con and SKO mice after 3 days of cold exposure as detected by qPCR (n = 8 for each group). (D) Expression of PGC1a and Ucp1 in BAT from Con and SKO mice after 3 days of cold exposure. (E) Expression of pan-adipocyte and thermogenic marker genes in iWAT from Con and SKO mice after 3 days of cold exposure (n = 8 for each group). (F) Expression of the PGC1a and Ucp1 in iWAT from Con and SKO mice after 3 days of cold exposure. (G) TEM imaging of BAT of Con and SKO mice after 3 days of cold exposure. (H) Profile of transcript levels of differentially expressed BAT-specific genes in Con and SKO mice after 3 days of cold exposure. ∗P < 0.05.
3.5. Survivin is essential for the thermogenic program and mitochondrial respiration in thermogenic adipocytes in vitro
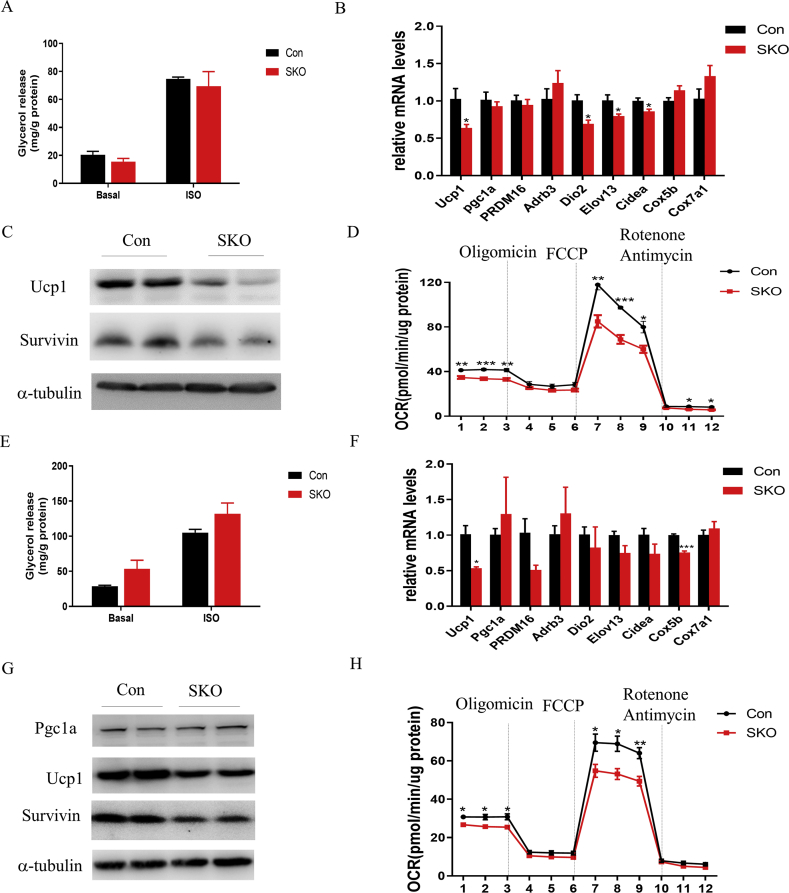
To examine the cell-autonomous effects of survivin on the differentiation and function of beige and brown adipocytes, SVFs of iWAT and BAT were isolated from SKO and Con mice and differentiated for 6 days. Survivin deletion in white, beige, and brown adipocytes did not affect the differentiation of cells and intracellular lipid accumulation, as determined by Oil Red O staining, light microscopy, and TG content analysis (Figure S4A, B, D, E, G, and H). Although the expression levels of the pan-adipocyte differentiation marker genes Fabp4 and Pparγ were slightly decreased in differentiated brown adipocytes from SKO mice, other differentiation marker genes (Cebpα and Adipoq) showed no change (Figure S4C). In addition, there was no difference in the expression of the abovementioned differentiation marker genes in differentiated beige adipocytes between Con and SKO mice (Figure S4F). The basal and ISO-stimulated lipolysis levels were not affected in either brown and beige adipocytes from SKO mice (Figure 5A,E). The expression levels of the classic thermogenic genes Ucp1, Dio2, Elov13, and cidea significantly decreased in brown adipocytes from SKO mice, yet neither Pgc-1α, encoding a transcriptional co-regulator of mitochondrial biogenesis, nor PRDM16 were affected (Figure 5B). Protein levels of Ucp1 also decreased upon survivin deletion in both brown and beige adipocytes (Figure 5C,G). Accordingly, both basal and maximum respiration rates were significantly decreased in both brown and beige adipocytes from SKO mice (Figure 5D,H). Collectively, our findings suggested that survivin deficiency represses the thermogenic program and mitochondrial respiration of thermogenic adipocytes in vitro.
Figure 5.
Survivin was essential for the thermogenic program and mitochondrial respiration of thermogenic adipocytes in vitro. (A) Glycerol release adjusted to cellular protein content under basal conditions or after treatment with 5 μmol/L isoproterenol (ISO) for 3 h in differentiated brown adipocytes from Con and SKO mice (n = 3). (B) Expression of thermogenic genes was detected by qRT-PCR in differentiated brown adipocytes from Con and SKO mice (n = 3). (C) Expression of survivin and the thermogenic protein Ucp1 in differentiated brown adipocytes from Con and SKO mice was determined by western blot. (D) Oxygen consumption rate (OCR) measurement of differentiated brown adipocytes from Con and SKO mice (n = 5). (E) Glycerol release adjusted to cellular protein content under basal conditions or after treatment with 5 μmol/L ISO for 3 h in differentiated beige adipocytes from Con and SKO mice (n = 4). (F) Expression of thermogenic genes in differentiated beige adipocytes from Con and SKO mice. (G) Expression of survivin, Pgc1α, and Ucp1 in differentiated beige adipocytes from Con and SKO mice. (H) OCR measurement of differentiated beige adipocytes from Con and SKO mice (n = 5). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
3.6. Adipocyte-specific deletion of survivin induces autophagy in vivo and in vitro
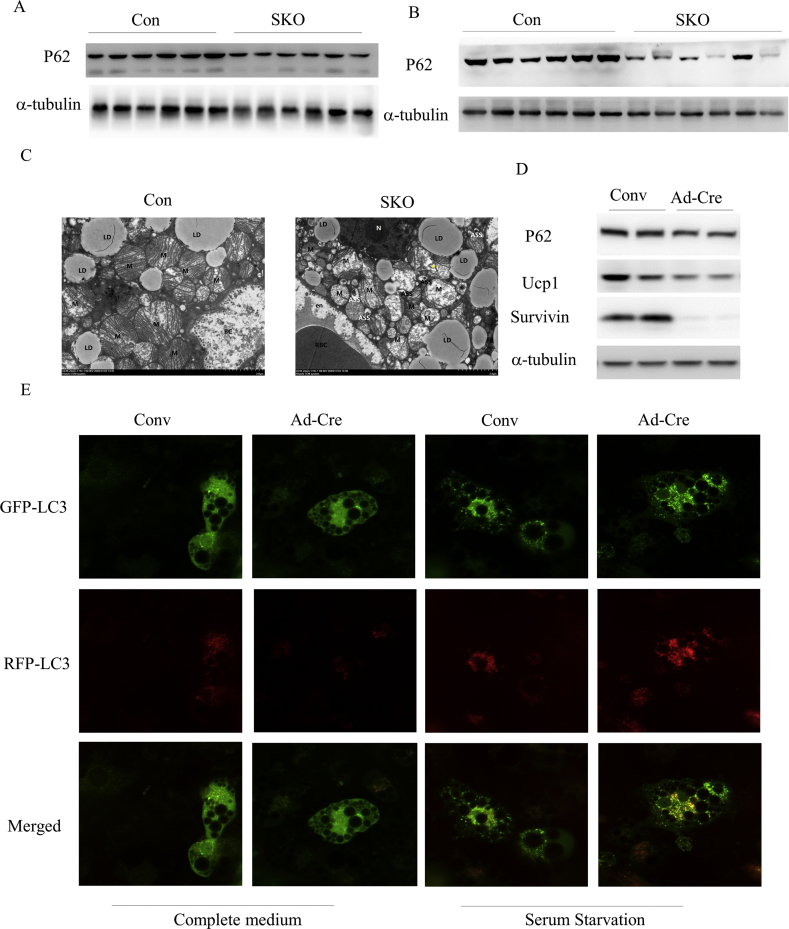
Previous studies indicated that survivin is a negative regulator of autophagy, which might be mediated via interacting proteins involved in autophagosome formation like beclin 1 and autophagy-related protein 5 (Atg5) [31,32]. Recent studies have reported that autophagy activity increased during beige-to-white adipocyte transition and BAT whitening [33,34]. Therefore, we further investigated whether survivin plays a role in maintaining BAT thermogenic function and homeostasis through regulating autophagy. SQSTM1/p62 is a cargo receptor protein that binds to ubiquitinated targets in the cytosol and is degraded in autolysosomes, thus serving as an index to monitor autophagic degradation [35]. The SQSTM1/p62 level was reduced in BAT of 24-week-old SKO mice fed CD (Figure 6 A), suggesting that autophagy was activated in SKO BAT under control conditions. Compared to Con mice, the SQSTM1/p62 protein levels were drastically decreased in SKO BAT after 3 days of cold exposure (Figure 6B). TEM analysis further confirmed autophagy was induced in SKO BAT, as the number of autolysosomes significantly increased upon cold exposure (Figure 6C). Consistent with this result, the protein level of SQSTM1/p62 was also reduced in survivin-deleted beige adipocytes treated with DMEM containing 0.2% BSA (Figure 6D). To capture the dynamic changes in autophagic activity induced by survivin deletion, we used mRFP-GFP-LC3 adenovirus to assess the levels of punctate LC3, which could indicate real-time autophagic flux. GFP puncta only detects autophagosomes, as pH-sensitive GFP could be easily quenched in the autolysosomes, and mRFP puncta indicates both autophagosomes and autolysosomes, as it is more stable in acidic conditions. We observed that both RFP-LC3 and GFP-LC3 puncta were increased in beige adipocytes after replacing 10% FBS complete medium with serum starvation medium containing 0.2% BSA for 2 h. Moreover, in survivin-deleted beige adipocytes, the GFP-LC3 puncta and the RFP-LC3 puncta were robustly increased with serum starvation medium compared to control, suggesting that autophagic flux was increased. Lastly, functional cluster analysis of upregulated DEGs revealed that various transportation and amino acid metabolic processes were among the most important functional groups in BAT of SKO mice (Figure S3H), which might be a downstream response of activated autophagy. These data collectively suggest that autophagic activity increased in survivin-depleted brown/beige adipocytes.
Figure 6.
Adipocyte-specific deletion of survivin induced autophagy in vivo and in vitro. (A) Protein expression of SQSTM1/p62 in BAT of 24-week-old Con and SKO mice fed CD. (B) Protein expression of SQSTM1/p62 in BAT of 9-week-old Con and SKO mice after 3 days of cold exposure. (C) Representative TEM imaging of BAT of Con and SKO mice after 3 days of cold exposure. (D) Immunoblotting for SQSTM1/p62 and Ucp1 of differentiated beige adipocytes from Survivinflox/flox mice infected with Conv or Ad-Cre and treated with 0.2% BSA for 4 h. (E) The iWAT SVFs were extracted from Survivinflox/flox mice, differentiated into beige adipocytes, and successively infected with Conv or Ad-Cre and Ad-mRFP-GFP-LC3. Images were acquired using a confocal laser scanning microscope after culturing cells in fresh complete DMEM containing 10% FBS or serum starvation medium supplemented 0.2% BSA for 2 h.
4. Discussion
Our previous work has demonstrated first that survivin expression could be induced in adipocytes upon long-term HFD feeding and that survivin plays a critical role in the protection against TNFα-induced adipocyte damage and lipolysis [22]. In the current study, we further show that survivin is upregulated in vitro upon treatment with BCAA, PA, or 3BDO, an activator of the mTORC1 pathway, and its expression is also induced by refeeding or short-term HFD feeding in adipose tissue in vivo; moreover, survivin is rapidly degraded after withdrawing FBS and glucose from the cell culture medium. In this study, we also discovered that adipocyte-specific deletion of survivin leads to reduced postnatal BAT mass and decreased Ucp1 protein expression. SKO mice also exhibited reduced thermogenic program and induced autophagic activity upon cold exposure. This subsequently impaired insulin sensitivity and glucose tolerance after HFD feeding. These results further reveal a previously unreported critical role for survivin in maintaining normal BAT mass, thermogenic program, and whole-body energy metabolism.
Although survivin expression in terminally differentiated mature adipocytes is low under basal conditions, our previous and current studies have demonstrated that survivin expression in mature adipocytes is highly nutrient-sensitive and that survivin could be re-expressed upon nutrient excess through activating the mTORC1 pathway; this indicates that survivin might participate in the response to various nutritional stresses in adipocytes. mTORC1 is a key energy sensor that not only influences the functions and metabolism of fat tissues but is also involved in thermogenesis in brown and beige adipocytes [11]. Studies in which the effects of adipose tissue-specific deletion of raptor, a key component of mTORC1, on WAT browning were analyzed led to discrepant results [[36], [37], [38], [39]], but decreased BAT mass and Ucp1 expression were consistently reported [[36], [37], [38]], emphasizing the requirement of mTORC1 for BAT formation and maintenance. To our knowledge, however, the role of survivin in BAT has never been explored. Firstly, we found that survivin expression is simultaneously upregulated upon thermogenic activation such as postprandial conditions or treatment with β3-adrenergic agonists, indicating survivin expression is positively associated with thermogenic activity. Secondly, Sun et al. have identified 10 adipocyte subpopulations via an integrated analysis of single-nucleus RNA-sequencing data of adipocyte nuclei from mice kept under room temperature, cold exposure, or thermoneutrality. Survivin is specifically abundant in a subpopulation from the cold exposure group [40]. Notably, adipose tissue-specific deletion of survivin has yielded similar phenotypes in BAT as raptor conditional knockout. Further investigation is needed to address whether survivin partially mediates the stimulatory effects of mTORC1 on BAT development and thermogenic functions.
As a member of the inhibitor of apoptosis family, the most common role of survivin is believed to be protection of cells against apoptotic and autophagic death [41]. However, survivin deletion in adipocytes did not significantly alter the activity of apoptosis pathway in adipose tissue (Figure S5A–D). In addition, adipocytes have been reported to have remarkable ability to resist apoptosis [42,43], and it has also been reported that the number of adipocytes remains constant in adulthood of lean and obese subjects, even after significant weight loss, indicating that the number of adipocytes is set before adulthood [44]. It has also been demonstrated that survivin is involved in stemness as survivin knockdown decreases the expression of key transcription factors, which is associated with pluripotency [45]. It appears that survivin plays a similar role in maintaining the intrinsic function of different types of adipocytes, i.e., energy storage in white adipocytes and energy dissipation through non-shivering thermogenesis in beige/brown adipocytes. Moreover, survivin localized in mitochondria could promote cellular respiration through cooperation with Hsp90 chaperones to enhance the stability of oxidative phosphorylation complex II in certain tumor cell lines [46]. One previous study revealed that liver mitochondria from survivin+/- mice have more defects in oxidative phosphorylation when treated with C2-Ceramide [47]. Accordingly, our results show that survivin knockout results in decreased mitochondrial respiration in thermogenic adipocytes, further supporting the notion that survivin might regulate mitochondrial oxidative phosphorylation, irrespective of cell type.
Many studies have indicated that survivin is a negative regulator of autophagy [[48], [49], [50]]. Autophagy plays a major role in the regulation of the cellular adaptive response to various physiological and pathological challenges [51]. Autophagy is also intimately connected to the regulation of adipocyte differentiation and function [52]. Several studies have found that autophagy is inhibited when the thermogenic function of brown/beige adipocytes is activated [53,54], and browning is induced by impaired autophagy in adipocytes [55]. Autophagy is also needed to convert beige adipocytes to white adipocytes after withdrawing stimuli like cold exposure or β3-adrenergic agonists and BAT whitening [33,34], further indicating that autophagic activity is reciprocally associated with thermogenic activity. Therefore, we hypothesize that survivin could regulate thermogenic activity through modulating autophagy in beige/brown adipocytes. Indeed, our results demonstrate that adipocyte-specific deletion of survivin increased autophagy activation in BAT, evidenced by reduced SQSTM1/p62 protein levels and increased autolysosomes. Although we have not investigated the precise molecular mechanisms by which survivin affects autophagic activity, the mechanisms behind how survivin regulates autophagy have been extensively studied in recent years. It has been revealed that survivin interacts with several proteins involved in autophagosome formation, like beclin 1 and LC3 [31,48,56]. Moreover, it has been demonstrated that survivin interacts with Atg5 and inhibits the conjugation between Atg12 and Atg5 as well [32,50]. Collectively, these findings indicate that survivin generally represses autophagy through interference with the development of autophagosomes.
One limitation of the current study is that our results might be insufficient to fully distinguish the specific role of survivin in mature adipocytes and its effect on adipocytes differentiation. Thus, using survivinflox/floxAdipoq-CreERT2 mice to delete the survivin in adult mice or at late stage of differentiation by tamoxifen would be more suitable. Another limitation of this study is that we address the function of survivin in adipocytes responding to stress conditions such as over-nutrition or cold exposure using adipocyte-SKO mice. Nonetheless, transgenic adipocyte-specific survivin overexpression mice should be used in future investigations to rigorously establish the role of survivin in vivo. The third limitation of our study is that it might be possible that survivin knockout leads to impaired metabolic phenotypes through modulating other pathways besides autophagy, which warrants further investigation, since survivin is a multi-functional small protein with various functions in different cell types and is involved in mitosis, mitochondrial migration, and angiogenesis [41,57].
In conclusion, we demonstrate that survivin expression in adipocytes is closely regulated by nutritional levels and show for the first time that survivin is essential for maintaining BAT mass and thermogenic program. The impaired metabolic phenotypes of adipocyte-specific deletion of survivin are associated with induced autophagy activity. Therefore, the findings suggest that survivin in adipose tissue could be therapeutically targeted to treat and prevent obesity and obesity-related metabolic diseases.
Funding
This study was supported by grants from the National Key Research and Development Project of China (2019YFA0904501) and the National Natural Science Foundation of China (No.81670778, No. 81974122, and No. 81974116).
Author contributions
MA carried out the research, analyzed the results, and wrote the manuscript. JS and SC performed the experiments and analyzed the data. JM and YX assisted with mouse phenotyping, and NB, SC, and FH assisted with animal experiments. JX assisted with the RNA-seq data analysis, and XM assisted in reviewing and revising the manuscript. YY designed the study, interpreted the results, wrote, and revised the manuscript.
Acknowledgements
The experiments of this study were mainly performed at the Shanghai Key Laboratory of Diabetes Mellitus, Shanghai Diabetes Institute. We appreciate the staff at the platform for their generous research support. The authors thank Professor Edward Conway (Center for Transgene Technology and Gene Therapy, Flanders Interuniversity Institute for Biotechnology, University of Leuven, B-3000 Leuven, Belgium), Dr. Lijian Hui (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences), and Dr. Junli Liu (Shanghai Key Laboratory of Diabetes Mellitus, Shanghai Diabetes Institute) for generously providing survivinflox/flox mice and Adiponectin-Cre+/- mice. We thank Professor Jiqiu Wang's team at Ruijin Hospital (Shanghai Jiaotong University School of Medicine) for kindly providing technical support on OCR Measurements. The authors would also like to thank the Duoease Scientific Service Center for excellent language editing service.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2022.101446.
Contributor Information
Xiaojing Ma, Email: maxiaojing@sjtu.edu.cn.
Ying Yang, Email: yangyingsh@sjtu.edu.cn.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Multimedia component 1
References
- 1.Crewe C., An Y.A., Scherer P.E. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. Journal of Clinical Investigation. 2017;127(1):74–82. doi: 10.1172/JCI88883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang M., Lin Y., Wang L., You X., Wang S., Zhao J., et al. Adipose tissue lipolysis is regulated by PAQR11 via altering protein stability of phosphodiesterase 4D. Molecular Metabolism. 2021;47:101182. doi: 10.1016/j.molmet.2021.101182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghaben A.L., Scherer P.E. Adipogenesis and metabolic health. Nature Reviews Molecular Cell Biology. 2019;20(4):242–258. doi: 10.1038/s41580-018-0093-z. [DOI] [PubMed] [Google Scholar]
- 4.Jonker J.W., Suh J.M., Atkins A.R., Ahmadian M., Li P., Whyte J., et al. A PPARgamma-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature. 2012;485(7398):391–394. doi: 10.1038/nature10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peirce V., Pellegrinelli V., Vidal-Puig A. In: Metabolic syndrome: a comprehensive textbook. Ahima R.S., editor. Springer International Publishing; Cham: 2016. Adipose structure (white, Brown, beige) pp. 369–396. [Google Scholar]
- 6.Wang Q.A., Tao C., Gupta R.K., Scherer P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nature Medicine. 2013;19(10):1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moser C., Straub L.G., Rachamin Y., Dapito D.H., Kulenkampff E., Ding L., et al. Quantification of adipocyte numbers following adipose tissue remodeling. Cell Reports. 2021;35(4):109023. doi: 10.1016/j.celrep.2021.109023. [DOI] [PubMed] [Google Scholar]
- 8.Kajimura S., Spiegelman B.M., Seale P. Brown and beige fat: physiological roles beyond heat generation. Cell Metabolism. 2015;22(4):546–559. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Reviews. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 10.Bartelt A., Heeren J. Adipose tissue browning and metabolic health. Nature Reviews Endocrinology. 2014;10(1):24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 11.Ye Y., Liu H., Zhang F., Hu F. mTOR signaling in Brown and Beige adipocytes: implications for thermogenesis and obesity. Nutrition and Metabolism. 2019;16:74. doi: 10.1186/s12986-019-0404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito M., Matsushita M., Yoneshiro T., Okamatsu-Ogura Y. Brown adipose tissue, diet-induced thermogenesis, and thermogenic food ingredients: from mice to men. Frontiers in Endocrinology. 2020;11:222. doi: 10.3389/fendo.2020.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beiroa D., Imbernon M., Gallego R., Senra A., Herranz D., Villarroya F., et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes. 2014;63(10):3346–3358. doi: 10.2337/db14-0302. [DOI] [PubMed] [Google Scholar]
- 14.Li Y., Schnabl K., Gabler S.M., Willershäuser M., Reber J., Karlas A., et al. Secretin-activated Brown fat mediates prandial thermogenesis to induce satiation. Cell. 2018;175(6):1561–1574. doi: 10.1016/j.cell.2018.10.016. e1512. [DOI] [PubMed] [Google Scholar]
- 15.Rafatmanesh A., Behjati M., Mobasseri N., Sarvizadeh M., Mazoochi T., Karimian M. The survivin molecule as a double-edged sword in cellular physiologic and pathologic conditions and its role as a potential biomarker and therapeutic target in cancer. Journal of Cellular Physiology. 2020;235(2):725–744. doi: 10.1002/jcp.29027. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda S., Pelus L.M. Survivin, a cancer target with an emerging role in normal adult tissues. Molecular Cancer Therapeutics. 2006;5(5):1087–1098. doi: 10.1158/1535-7163.MCT-05-0375. [DOI] [PubMed] [Google Scholar]
- 17.Deguchi M., Shiraki K., Inoue H., Okano H., Ito T., Yamanaka T., et al. Expression of survivin during liver regeneration. Biochemical and Biophysical Research Communications. 2002;297(1):59–64. doi: 10.1016/s0006-291x(02)02128-9. [DOI] [PubMed] [Google Scholar]
- 18.Chiou S.K., Moon W.S., Jones M.K., Tarnawski A.S. Survivin expression in the stomach: implications for mucosal integrity and protection. Biochemical and Biophysical Research Communications. 2003;305(2):374–379. doi: 10.1016/s0006-291x(03)00724-1. [DOI] [PubMed] [Google Scholar]
- 19.Altznauer F., Martinelli S., Yousefi S., Thurig C., Schmid I., Conway E.M., et al. Inflammation-associated cell cycle-independent block of apoptosis by survivin in terminally differentiated neutrophils. Journal of Experimental Medicine. 2004;199(10):1343–1354. doi: 10.1084/jem.20032033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ejarque M., Ceperuelo-Mallafre V., Serena C., Pachon G., Nunez-Alvarez Y., Terron-Puig M., et al. Survivin, a key player in cancer progression, increases in obesity and protects adipose tissue stem cells from apoptosis. Cell Death & Disease. 2017;8(5) doi: 10.1038/cddis.2017.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izquierdo A.G., Carreira M.C., Rodriguez-Carnero G., Fernandez-Quintela A., Sueiro A.M., Martinez-Olmos M.A., et al. Weight loss normalizes enhanced expression of the oncogene survivin in visceral adipose tissue and blood leukocytes from individuals with obesity. International Journal of Obesity. 2021;45(1):206–216. doi: 10.1038/s41366-020-0630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ju L., Zhang X., Deng Y., Han J., Yang J., Chen S., et al. Enhanced expression of Survivin has distinct roles in adipocyte homeostasis. Cell Death & Disease. 2017;8(1) doi: 10.1038/cddis.2016.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Q., Zhang Z., Rong W., Jin W., Yan L., Jin W., et al. KMT5c modulates adipocyte thermogenesis by regulating Trp53 expression. Proceedings of the National Academy of Sciences of the U S A. 2020;117(36):22413–22422. doi: 10.1073/pnas.1922548117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xing Z., Conway E.M., Kang C., Winoto A. Essential role of survivin, an inhibitor of apoptosis protein, in T cell development, maturation, and homeostasis. Journal of Experimental Medicine. 2004;199(1):69–80. doi: 10.1084/jem.20031588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai N., Ma J., Alimujiang M., Xu J., Hu F., Xu Y., et al. Bola3 regulates beige adipocyte thermogenesis via maintaining mitochondrial homeostasis and lipolysis. Frontiers in Endocrinology. 2020;11:592154. doi: 10.3389/fendo.2020.592154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon M.S., Sun Y., Arauz E., Jiang Y., Chen J. Phosphatidic acid activates mammalian target of rapamycin complex 1 (mTORC1) kinase by displacing FK506 binding protein 38 (FKBP38) and exerting an allosteric effect. Journal of Biological Chemistry. 2011;286(34):29568–29574. doi: 10.1074/jbc.M111.262816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ge D., Han L., Huang S., Peng N., Wang P., Jiang Z., et al. Identification of a novel MTOR activator and discovery of a competing endogenous RNA regulating autophagy in vascular endothelial cells. Autophagy. 2014;10(6):957–971. doi: 10.4161/auto.28363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao J., Tenev T., Martins L.M., Downward J., Lemoine N.R. The ubiquitin-proteasome pathway regulates survivin degradation in a cell cycle-dependent manner. Journal of Cell Science. 2000;113 Pt 23:4363–4371. doi: 10.1242/jcs.113.23.4363. [DOI] [PubMed] [Google Scholar]
- 29.Hagenbuchner J., Kiechl-Kohlendorfer U., Obexer P., Ausserlechner M.J. BIRC5/Survivin as a target for glycolysis inhibition in high-stage neuroblastoma. Oncogene. 2016;35(16):2052–2061. doi: 10.1038/onc.2015.264. [DOI] [PubMed] [Google Scholar]
- 30.Alvarez-Dominguez J.R., Bai Z., Xu D., Yuan B., Lo K.A., Yoon M.J., et al. De novo reconstruction of adipose tissue transcriptomes reveals long non-coding RNA regulators of Brown adipocyte development. Cell Metabolism. 2015;21(5):764–776. doi: 10.1016/j.cmet.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niu T.K., Cheng Y., Ren X., Yang J.M. Interaction of Beclin 1 with survivin regulates sensitivity of human glioma cells to TRAIL-induced apoptosis. FEBS Letters. 2010;584(16):3519–3524. doi: 10.1016/j.febslet.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maskey D., Yousefi S., Schmid I., Zlobec I., Perren A., Friis R., et al. ATG5 is induced by DNA-damaging agents and promotes mitotic catastrophe independent of autophagy. Nature Communications. 2013;4:2130. doi: 10.1038/ncomms3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altshuler-Keylin S., Shinoda K., Hasegawa Y., Ikeda K., Hong H., Kang Q., et al. Beige adipocyte maintenance is regulated by autophagy-induced mitochondrial clearance. Cell Metabolism. 2016;24(3):402–419. doi: 10.1016/j.cmet.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng J., Guo Y., Yuan F., Chen S., Yin H., Jiang X., et al. Autophagy inhibition prevents glucocorticoid-increased adiposity via suppressing BAT whitening. Autophagy. 2020;16(3):451–465. doi: 10.1080/15548627.2019.1628537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klionsky D.J., Abdel-Aziz A.K., Abdelfatah S., Abdellatif M., Abdoli A., Abel S., et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)(1) Autophagy. 2021;17(1):1–382. doi: 10.1080/15548627.2020.1797280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Labbé S.M., Mouchiroud M., Caron A., Secco B., Freinkman E., Lamoureux G., et al. mTORC1 is required for Brown adipose tissue recruitment and metabolic adaptation to cold. Scientific Reports. 2016;6:37223. doi: 10.1038/srep37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu D., Bordicchia M., Zhang C., Fang H., Wei W., Li J.L., et al. Activation of mTORC1 is essential for β-adrenergic stimulation of adipose browning. Journal of Clinical Investigation. 2016;126(5):1704–1716. doi: 10.1172/JCI83532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X., Luo Y., Wang C., Ding X., Yang X., Wu D., et al. Adipose mTORC1 suppresses prostaglandin signaling and beige adipogenesis via the CRTC2-COX-2 pathway. Cell Reports. 2018;24(12):3180–3193. doi: 10.1016/j.celrep.2018.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shan T., Zhang P., Jiang Q., Xiong Y., Wang Y., Kuang S. Adipocyte-specific deletion of mTOR inhibits adipose tissue development and causes insulin resistance in mice. Diabetologia. 2016;59(9):1995–2004. doi: 10.1007/s00125-016-4006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun W., Dong H., Balaz M., Slyper M., Drokhlyansky E., Colleluori G., et al. snRNA-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Nature. 2020;587(7832):98–102. doi: 10.1038/s41586-020-2856-x. [DOI] [PubMed] [Google Scholar]
- 41.Wheatley S.P., Altieri D.C. Survivin at a glance. Journal of Cell Science. 2019;132(7) doi: 10.1242/jcs.223826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Magun R., Boone D.L., Tsang B.K., Sorisky A. The effect of adipocyte differentiation on the capacity of 3T3-L1 cells to undergo apoptosis in response to growth factor deprivation. International Journal of Obesity and Related Metabolic Disorders. 1998;22(6):567–571. doi: 10.1038/sj.ijo.0800626. [DOI] [PubMed] [Google Scholar]
- 43.Xiao Y., Yuan T., Yao W., Liao K. 3T3-L1 adipocyte apoptosis induced by thiazolidinediones is peroxisome proliferator-activated receptor-gamma-dependent and mediated by the caspase-3-dependent apoptotic pathway. FEBS Journal. 2010;277(3):687–696. doi: 10.1111/j.1742-4658.2009.07514.x. [DOI] [PubMed] [Google Scholar]
- 44.Spalding K.L., Arner E., Westermark P.O., Bernard S., Buchholz B.A., Bergmann O., et al. Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 45.Mull A.N., Klar A., Navara C.S. Differential localization and high expression of SURVIVIN splice variants in human embryonic stem cells but not in differentiated cells implicate a role for SURVIVIN in pluripotency. Stem Cell Research. 2014;12(2):539–549. doi: 10.1016/j.scr.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Rivadeneira D.B., Caino M.C., Seo J.H., Angelin A., Wallace D.C., Languino L.R., et al. Survivin promotes oxidative phosphorylation, subcellular mitochondrial repositioning, and tumor cell invasion. Science Signaling. 2015;8(389):ra80. doi: 10.1126/scisignal.aab1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conway E.M., Pollefeyt S., Steiner-Mosonyi M., Luo W., Devriese A., Lupu F., et al. Deficiency of survivin in transgenic mice exacerbates Fas-induced apoptosis via mitochondrial pathways. Gastroenterology. 2002;123(2):619–631. doi: 10.1053/gast.2002.34753. [DOI] [PubMed] [Google Scholar]
- 48.Roca H., Varsos Z., Pienta K.J. CCL2 protects prostate cancer PC3 cells from autophagic death via phosphatidylinositol 3-kinase/AKT-dependent survivin up-regulation. Journal of Biological Chemistry. 2008;283(36):25057–25073. doi: 10.1074/jbc.M801073200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ulasov I.V., Tyler M.A., Zhu Z.B., Han Y., He T.C., Lesniak M.S. Oncolytic adenoviral vectors which employ the survivin promoter induce glioma oncolysis via a process of beclin-dependent autophagy. International Journal of Oncology. 2009;34(3):729–742. doi: 10.3892/ijo_00000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin T.Y., Chan H.H., Chen S.H., Sarvagalla S., Chen P.S., Coumar M.S., et al. BIRC5/Survivin is a novel ATG12-ATG5 conjugate interactor and an autophagy-induced DNA damage suppressor in human cancer and mouse embryonic fibroblast cells. Autophagy. 2019:1–18. doi: 10.1080/15548627.2019.1671643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaur J., Debnath J. Autophagy at the crossroads of catabolism and anabolism. Nature Reviews Molecular Cell Biology. 2015;16(8):461–472. doi: 10.1038/nrm4024. [DOI] [PubMed] [Google Scholar]
- 52.Lahiri V., Hawkins W.D., Klionsky D.J. Watch what you (self-) eat: autophagic mechanisms that modulate metabolism. Cell Metabolism. 2019;29(4):803–826. doi: 10.1016/j.cmet.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cairó M., Villarroya J., Cereijo R., Campderrós L., Giralt M., Villarroya F. Thermogenic activation represses autophagy in brown adipose tissue. International Journal of Obesity. 2016;40(10):1591–1599. doi: 10.1038/ijo.2016.115. [DOI] [PubMed] [Google Scholar]
- 54.Cairó M., Campderrós L., Gavaldà-Navarro A., Cereijo R., Delgado-Anglés A., Quesada-López T., et al. Parkin controls brown adipose tissue plasticity in response to adaptive thermogenesis. EMBO Reports. 2019;20(5) doi: 10.15252/embr.201846832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martinez-Lopez N., Athonvarangkul D., Sahu S., Coletto L., Zong H., Bastie C.C., et al. Autophagy in Myf5+ progenitors regulates energy and glucose homeostasis through control of brown fat and skeletal muscle development. EMBO Reports. 2013;14(9):795–803. doi: 10.1038/embor.2013.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Humphry N.J., Wheatley S.P. Survivin inhibits excessive autophagy in cancer cells but does so independently of its interaction with LC3. Biol Open. 2018;7(10) doi: 10.1242/bio.037374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanhueza C., Wehinger S., Castillo Bennett J., Valenzuela M., Owen G.I., Quest A.F.G. The twisted survivin connection to angiogenesis. Molecular Cancer. 2015;14(1) doi: 10.1186/s12943-015-0467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1