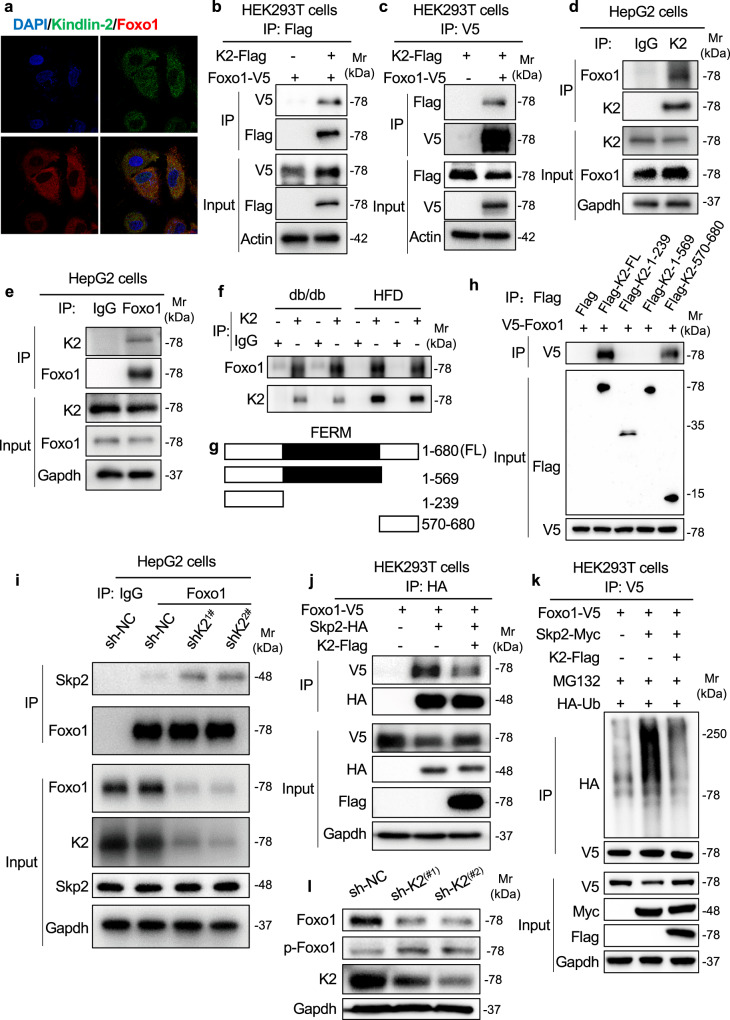
Fig. 6. Kindlin-2 interacts with Foxo1 and inhibits Foxo1 ubiquitination through E3 ligase Skp2.
a IF staining. Huh7 cells were subjected to double immunostaining with anti-Foxo1 antibody (red) and anti-Kindlin-2 antibody (green), followed by visualization with confocal microscopy. Scale bar, 20 μm. b, c co-IP assays. Cell lysates from HEK293T cells transfected with V5-tagged Foxo1 with and without Flag-Kindlin-2 were used for IP and IB assays. d, e co-IP assays. HepG2 cells were used for IP and IB with the indicated antibodies for the interaction of endogenous Kindlin-2 and Foxo1. f The lysates from livers of HFD-fed mice and db/db mice were prepared and subjected to IP and IB assays. g A schematic diagram of the full-length (FL) and truncated Kindlin-2 plasmid constructs. h co-IP assays. HEK293T cells were co-transfected with plasmid constructs expressing Foxo1 and full-length or truncated Kindlin-2. Forty-eight hours later, whole-cell extracts were prepared and subjected to co-IP assays. i Kindlin-2 KD increases the interaction of Skp2 and Foxo1. Cell lysates from HepG2 cells infected with lentiviruses expressing Kindlin-2 shRNA (sh-K2, #1 and #2) or negative control shRNA (sh-NC) were subjected to IP and IB assays. j Kindlin-2 OE reduces the interaction of Skp2 and Foxo1. HEK293T cells transfected with the indicated plasmids were subjected to IP, followed by IB using the indicated antibodies. k Foxo1 ubiquitination. HEK293T cells were transfected with the indicated plasmids. Forty-eight hours after transfection, the cells were pretreated with MG132 (10 μM) for 6 h, followed by IP and IB assays. l Western blotting. Huh7 cells were infected with lentiviruses-expressed control shRNA (sh-NC) and two different Kindlin-2 shRNAs (sh-K2(#1), sh-K2(#2)). After 96 h, cells were harvested, followed by an IB assay. For b–h, j, k, 200 μg of whole-cell extracts from each group were used for IP assays. Immunoprecipitates were resuspended in 50 μl buffer. Fifteen microlitres from each sample was loaded for SDS-PAGE, followed by IB assays. Protein extracts (20 μg) were used for western blotting from each input sample for (b–e, h–l) (bottom input panels). a–f, h–l Data are representative of three biologically independent replicates.