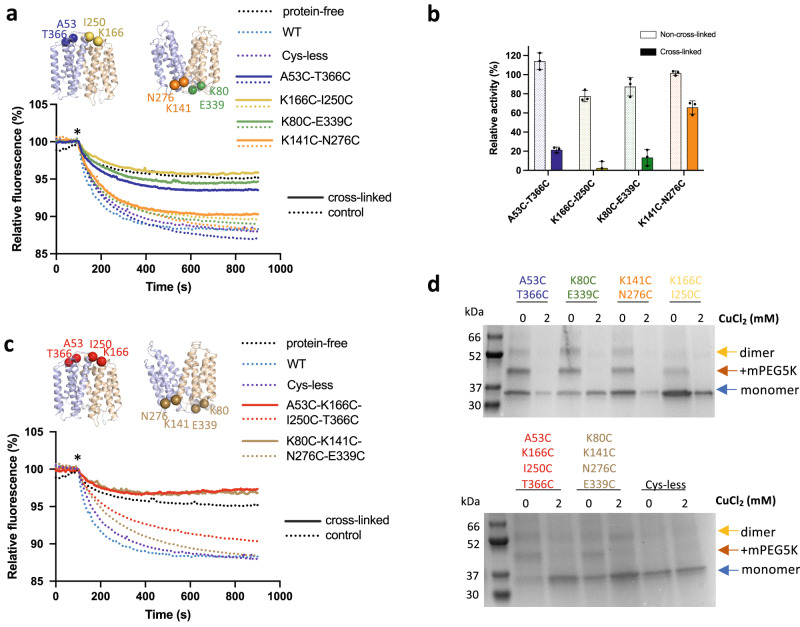
Fig. 4. Cycling through outward- and inward-facing conformations is essential for LtaA activity.
a, c Proton-transport activity of LtaA and variants after chemical cross-linking with CuCl2 (solid lines) or in absence of cross-linking treatment (dotted lines) (n ≥ 3). Proteoliposomes and protein-free liposomes containing 100 mM KCl were diluted in buffer containing 10 mM KCl, 90 mM NaCl, and ACMA. H+ influx was initiated by establishing a membrane potential upon the addition of the potassium ionophore valinomycin (asterisk). b Relative activity of cross-linked and non-cross-linked LtaA variants measured in A. Relative activity = 100 × (F’i − F’liposomes)/(F’wt − F’liposomes), where i corresponds to each variant, liposomes correspond to protein-free liposomes, and F’ correspond to the relative fluorescence at the plateau (800 s). Error bars show + /− s.d. of technical replicates, n = 3. d SDS-PAGE shows band shifts of proteoliposome samples treated with mPEG5K after cross-linking with CuCl2. Separated species are indicated with arrows. SDS-PAGE experiments were independently repeated at least three times with similar results. Source data are provided as a Source Data file.