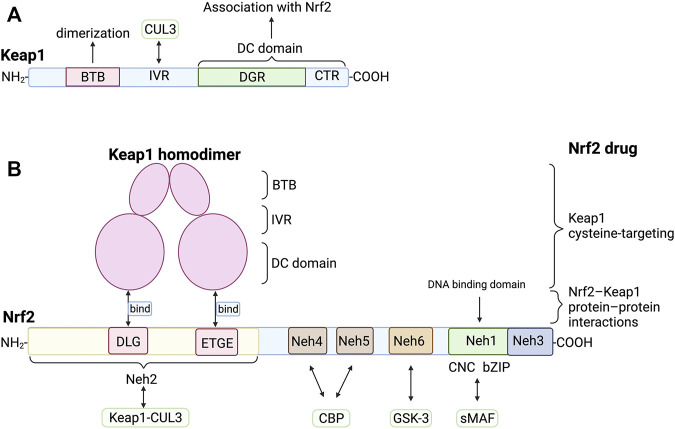
FIGURE 2.
Structure and interaction of Keap1 and Nrf2 (A) Keap1 contains BTB domain, IVR domain, DGR domain and CTR domain. Its dimerization is mediated by BTB domain. IVR domain and CUL3 interact to form Keap1-CUL3 complex, which ubiquitinates Nrf2 under normal condition. The DGR and CTR domain are collectively called DC domain, which directly interact with the DLG and ETGE of Neh2 of Nrf2. (B) The DLG and ETGE domain on Neh2 could bind with DC domain of Keap1 homodimer. sMAF can bind with Neh1 of Nrf2 to form heterodimers that bind to DNA. Neh6 could be phosphorylated by GSK-3, leading to the degradation of Nrf2. There are several types of Nrf2 activators, such as Keap1 cysteine-targeting drugs and drugs that disrupt the Nrf2-Keap1 protein-protein interactions. BTB, bric-a-brac domain; CBP, cAMP responsive element binding protein; CTR, carboxyl terminal region; CUL3, CULLIN3; DGR, double glycine repeat; GSK-3, glycogen synthase kinase 3; IVR, intermediate region; Neh1, Nrf2-ECH homology domain-1; sMAF, small musculoaponeurotic fibrosarcoma oncogene homologue.