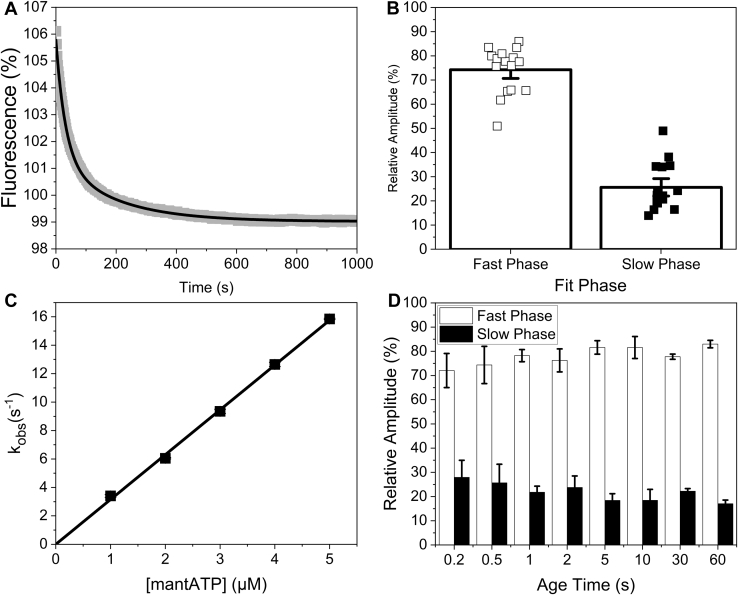
Figure 1.
MantATP association with bovine masseter myofibrils.A, an example transient of 100/50 nM bovine masseter myofibrils incubate with 10/5 μM mant-ATP for 1 min then rapidly mixed with 250/125 μM MgATP in the stopped flow. This was best described by a double exponential, resulting in a kobs = 0.027 s−1 and 0.0038 s−1 for the fast and slow phase respectively and an amplitude of 5.6% and 1.6% for the fast and slow phase, respectively. B, relative amplitudes of the fast (DRX, open squares) and slow (SRX, closed squares) phases of mant-ATP displacement from bovine masseter myofibrils at 20 °C. C, plot of the observed rate constant of mant-ATP binding to bovine masseter myofibrils using single mixing. The second-order on-rate constant derived from the slope (K1k+2) = 3.2 ± 0.03 μM−1 s−1. D, the relative amplitudes of double mixing assays. 100/25 nM bovine masseter myofibrils rapidly mixed with 10/2.5 μM mant-ATP for different age times (0.2–60 s), then rapidly mixed with 250/125 μM MgATP.