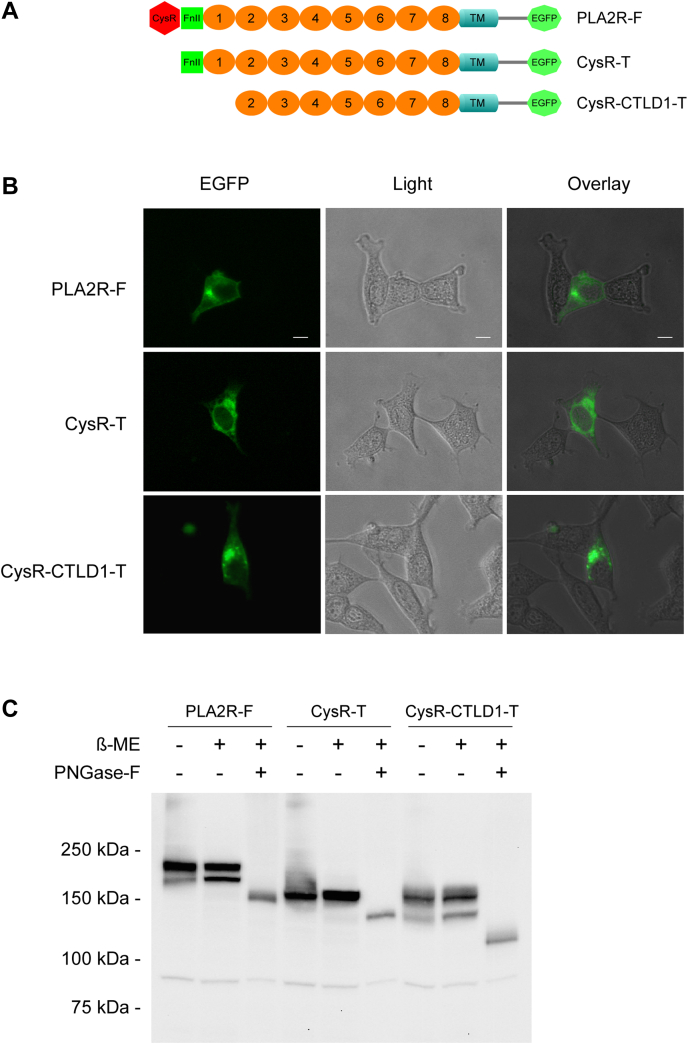
Figure 2.
Surface expression and deglycosylation study of PLA2R N-terminal domain truncations.A, schematic illustration of the EGFP-fused PLA2R constructs. B, HEK 293 cells expressing PLA2R full-length, CysR, or CysR-CTLD1 domain truncations were plated at a low density on polylysine coated coverslips, fixed with 4% paraformaldehyde (pH 7.4) and imaged under a fluorescence microscope. The scale bar indicates 10 μm. C, cell lysates from transfected HEK 293 cells were treated with PNGase-F following the manufacturer’s instruction. Protein samples were resolved by 7.5% SDS-PAGE, transferred to a nitrocellulose membrane, and probed with a rabbit anti-human PLA2R polyclonal antibody. Each experiment was performed 3 to 5 times. -, without β-ME or PNGase-F; +, with β-ME or PNGase-F; β-ME, β-Mercaptoethanol; Anti-PLA2R-Ab, rabbit anti-human PLA2R polyclonal antibody; CTLD, C-type lectin-like domain; CysR, cysteine-rich domain; CysR-CTLD1-T, CysR-CTLD1 triple domain truncation; CysR-T, CysR domain truncation; EGFP, enhanced green fluorescent protein; PLA2R, phospholipase A2 receptor; PLA2R-F, PLA2R full-length; PNGase-F, peptide N-glycosidase F.