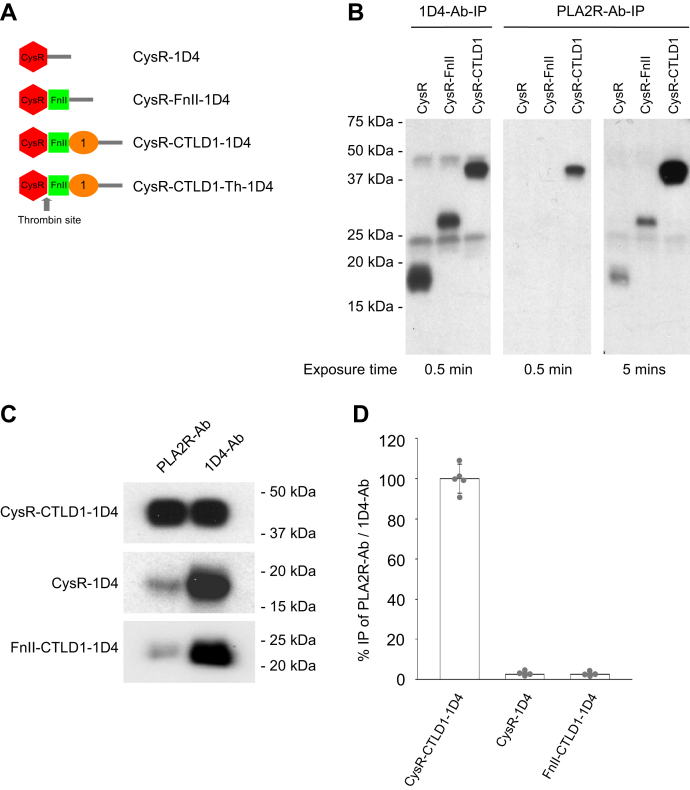
Figure 5.
Characterization of PLA2R-Ab interaction with PLA2R domain fragments under physiological conditions.A, schematic illustration of the 1D4-tagged PLA2R extracellular domain fragments. B, phospholipase A2 receptor N-terminal 1D4-tagged domain fragments, CysR, CysR-FnII, and CysR-CTLD1 were individually mixed with either the 1D4-Ab or the PLA2R-Ab positive serum in 500 μl TBS (pH 7.4) and incubated for 2 h at room temperature followed with protein G-sepharose resin immunoprecipitation. The immunoprecipitated proteins were eluted with SDS-sample buffer containing β-ME. The protein samples were resolved by 4 to 20% SDS-PAGE, transferred to nitrocellulose membrane, and probed by the 1D4-Ab. The film was exposed for 0.5 min and 5 min to detect the immunoprecipitated protein fragments. The experiment was performed three times. C, the 1D4-tagged CysR-CTLD1, CysR, and FnII-CTLD1 domain fragments (released from CysR-CTLD1-Th by thrombin digestion) were individually immunoprecipitated with the patient serum or 1D4-Ab in TBS buffer (pH 7.4) and further processed as described above. D, the blots were scanned and quantified using densitometry. The error bars represent mean ± S.D. (n = 5). 1D4-Ab-IP, immunoprecipitation with the anti-1D4 antibody; CTLD, C-type lectin-like domain; CysR, cysteine-rich domain; FnII, fibronectin type II domain; PLA2R, phospholipase A2 receptor; PLA2R-Ab-IP, immunoprecipitation with patient serum.