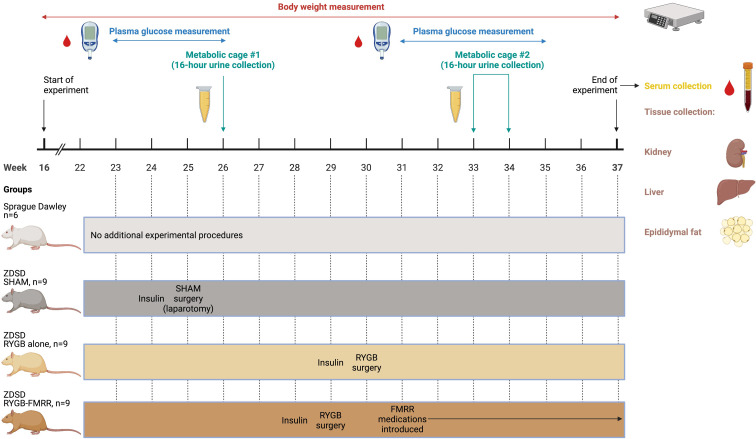
Figure 1.
Overview of the study design. Created with BioRender.com. In the SHAM, RYGB, and RYGB-FMRR groups, glycaemic control was optimized for one week prior to surgery with daily subcutaneous injection of insulin degludec (Tresiba®, Novo Nordisk) to achieve a fasting plasma glucose below 12 mmol/L. When introducing medications in the RYGB-FMRR group, metformin monotherapy was introduced for the first two days to monitor for adverse responses, including anorexia. The remaining medications (fenofibrate, ramipril, and rosuvastatin) were commenced thereafter when no adverse response was observed. FMRR, fenofibrate, metformin, ramipril, and rosuvastatin; RYGB, Roux-en-Y gastric bypass; RYGB-FMRR, Roux-en-Y gastric bypass plus fenofibrate, metformin, ramipril, and rosuvastatin; SHAM, sham surgery (laparotomy); ZDSD, Zucker Diabetic Sprague Dawley.