Abstract
In addition to genetic factors, environmental factors such as viruses are thought to be triggers in the development of autoimmune thyroid diseases (AITD) such as Graves' disease (GD). In this context, AITD cases that may be associated with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or immunization have begun to be reported in increasing numbers. Although it is not clear by which pathogenetic mechanisms immunization against coronavirus disease 2019 (COVID-19) triggers the development of AITD, both the potential effect of the adjuvants in the vaccines and the cross-reactivity that can be generated by the molecular similarity of viral particles with mammalian proteins seem to be possible mechanisms. In this article, 7 GD patients consisting of relapsed and newly diagnosed cases following the COVID-19 vaccination were presented. Of these 7 cases, 5 (71.4%) were female, and the median age of the patients was 47 years (range, 31–53). One of the patients was associated with the inactivated COVID-19 vaccine, while the others were associated with the mRNA COVID-19 vaccine. The median post-vaccination symptom onset was 7 days (range, 4–30). Three of the patients had a history of GD and one had a history of Hashimoto's thyroiditis. Rapidly developing Graves' ophthalmopathy was detected in one patient. These cases are cautionary that GD and its extrathyroidal manifestations may develop in a short period after COVID-19 vaccination. When considered together with the literature review, the history of AITD in approximately half of the patients suggests that more attention should be paid to these patients in the post-vaccination period. Nevertheless, multicenter, prospective studies are needed to better understand this possible causal relationship.
Keywords: Graves' disease, Autoimmune thyroid diseases, Hyperthyroidism, SARS-CoV-2, Inactivated COVID-19 vaccine, mRNA vaccine
1. Introduction
Vaccines developed to control the coronavirus disease 2019 (COVID-19) pandemic, which has profoundly shaken the whole world, are promising in terms of ending this pandemic. However, side-effects that may be associated with increased vaccination at unprecedented levels have also begun to be carefully monitored by clinicians.
Although the factors that play a role in the development of Graves’ disease (GD) have not been fully elucidated, it is thought that genetic, epigenetic, and environmental factors may be the initiator [1]. In particular, the hypothesis that certain common viral infections (such as Epstein-Barr virus, influenza virus) trigger GD by causing epigenetic regulations in genes associated with GD susceptibility (such as thyroglobulin, thyrotropin receptor gene) is one of the potential mechanisms [1]. In addition, the molecular mimicry between the virus and various human antigens is thought to induce autoimmunity by causing cross-reactivity [2]. From this point of view, the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection-associated autoimmune thyroid diseases (AITD) can be expected. Indeed, some cases of GD and other AITD have been reported following SARS-CoV-2 infection and its immunization [[3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]]. When considering the pre-COVID-19 period, in a case report published after the H1N1 pandemic, two newly-diagnosed autoimmune diseases (GD and narcolepsy type 1) had been detected following H1N1 vaccination in a 40-year-old female patient [14].
In this paper, 7 COVID-19 vaccine-associated GD cases are presented and the literature is reviewed. In addition, to the best of our knowledge, the current study describes the first case of inactivated COVID-19 vaccine-associated GD in the literature.
2. Methods
The literature search was conducted in order to identify COVID-19 vaccine-related GD case reports or series published until the end of December 2021 from the PubMed online database, using the following search string: (‘'Graves'' OR ″thyroiditis'' OR ″thyrotoxicosis'') AND (‘'COVID-19″ OR ″SARS-CoV-2″) AND (‘'vaccine'' OR ″immunization'').
The descriptive analysis was conducted using SPSS software (version 23.0, SPSS, IBM Corporation, NY, USA). Categorical data were expressed as frequencies and percentages (%). The median (range) was calculated for continuous variables.
3. Results
3.1. Relapsed Graves' disease
3.1.1. Patient 1
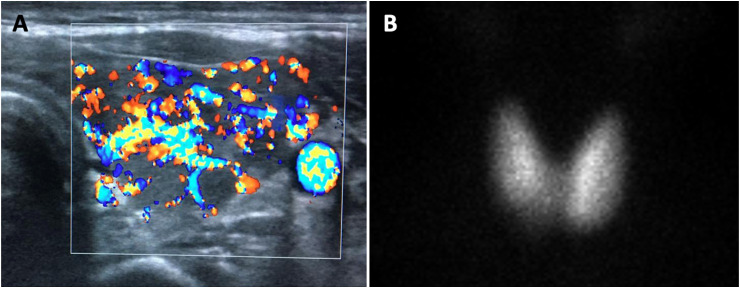
A 44-year-old female patient was diagnosed with GD 13 years ago and received treatment for 1 year. She was followed in remission after discontinuation of antithyroid treatment. There has been no follow up for the last 1.5 years due to the pandemic and the patient has not had a documented SARS-CoV-2 infection. One week after receiving the first dose of inactivated COVID-19 vaccine (CoronaVac®) on June 10, 2021, complaints of excessive sweating, palpitation, and fatigue started. After a 2-month delayed admission, the physical examination revealed an enlarged thyroid gland with palpation. Thyroid hormone and antibody panel results showed thyroid-stimulating hormone (TSH) < 0.01 mIU/L (0.27–4.2), free thyroxine (fT4):2.67 ng/dL (0.93–1.7), free triiodothyronine (fT3): 9.65 ng/L (2–4.4), anti-thyroid peroxidase (anti-TPO):284 IU/ml (0–34), anti-thyroglobuline (anti-Tg):119 IU/ml (0–115), and TSH receptor antibody (TRAb):12.18 IU/L (<1.5). Thyroid ultrasonography (US) showed hypoechoic areas separated by fibrous septa and increased parenchymal vascularity in a ‘Thyroid inferno’ pattern with Doppler (Fig. 1 A). With the diagnosis of relapsed GD, treatment with 20 mg/day methimazole (MMI) and propranolol was initiated.
Fig. 1.
(A) Patient 1: Doppler ultrasonography image of the left thyroid lobe consistent with the ‘Thyroid inferno’ pattern on the transverse axis, (B) Patient 4: scintigraphy image showing bilateral diffuse increased technetium pertechnetate uptake with suppressing background activity in the thyroid gland in anterior pinhole view.
3.1.2. Patient 2
A 49-year-old male patient who was diagnosed with GD in December 2018 is being followed up routinely in our outpatient clinic. After 18 months of antithyroid treatment, TRAb became negative and the treatment was discontinued. The patient, who was asymptomatic in the follow-up one year after the drug discontinuation in June 2021, was euthyroid. He had not had a documented SARS-CoV-2 infection. The patient, who received two doses of mRNA COVID-19 vaccine (Pfizer-BioNTech®) in the middle of June and July 2021, presented at our clinic again with complaints of palpitations, hand tremors, and sweating that started approximately 1 month after the second dose of vaccination. Thyroid hormone and antibody panel results were TSH<0.01 mIU/L (0.27–4.2), fT4:3.86 ng/dL (0.93–1.7), fT3: 13.50 ng/L (2–4.4), anti-TPO:435 IU/ml (0–34), anti-Tg:236 IU/ml (0–115), and TRAb:3.01 IU/L (<1.5). A moderate-to-severe increase in parenchymal vascularity of the thyroid gland was observed with Doppler US. With the diagnosis of relapsed GD, 20 mg/day MMI and propranolol were started. After 1 month, the fT4 level decreased to 1.45 ng/dL and fT3 decreased to 5.11 ng/L.
3.1.3. Patient 3
A 31-year-old female patient, who was diagnosed with GD in our outpatient clinic in January 2019, is also being followed up for breast cancer in remission. After 19 months of antithyroid treatment, TRAb became negative and her treatment was discontinued. At the control examination in June 2021, the patient was found to be asymptomatic and euthyroid. In addition, she had not had a documented SARS-CoV-2 infection. The patient, who had the first dose of mRNA COVID-19 vaccine (Pfizer-BioNTech®) at the beginning of August 2021, presented at our clinic with complaints of hot flushes, weakness, and sweating that had started approximately 3 weeks after the vaccination. Thyroid hormone and antibody panel results were TSH<0.01 mIU/L (0.27–4.2), fT4>7.77 ng/dL (0.93–1.7), fT3: 21.70 ng/L (2–4.4), anti-TPO: 325 IU/ml (0–34), anti-Tg:11 IU/ml (0–115), and TRAb:19.30 IU/L (<1.5). A moderate increase in parenchymal vascularity of the thyroid gland was observed with Doppler US. With the diagnosis of relapsed GD, 20 mg/day MMI and propranolol were started. After 5 weeks, the fT4 level decreased to 1.43 ng/dL and fT3 decreased to 6.19 ng/L.
3.2. Conversion from Hashimoto's thyroiditis to Graves' disease
3.2.1. Patient 4
A 53-year-old female patient who was diagnosed with Hashimoto's thyroiditis in September 2019 has been followed up with levothyroxine (LT) replacement for 2 years. The patient, who had her last visit before vaccination in April 2021, was found to be euthyroid with 50 mcg/day LT. She was infected with SARS-CoV-2 in August 2020. The patient, who received the first dose of mRNA COVID-19 vaccine (Pfizer-BioNTech®) in the middle of June 2021, and the second dose 1 month later, started to complain of palpitations, sweating, and weight loss 1 week after the first dose of vaccination. While continuing the LT treatment during this period, the patient presented again at our outpatient clinic in October 2021. The thyroid hormone panel results were TSH<0.01 mIU/L (0.27–4.2), fT4: 4.01 ng/dL (0.93–1.7), fT3: 8.83 ng/L (2–4.4). When LT replacement was stopped and re-evaluated 12 days later, fT4: 2.95 ng/dL, fT3: 9.05 ng/L, Tg: 1.32 ng/mL (3.68–64.15), anti-TPO: 55 IU/ml (0–34), anti-Tg: 1197 IU/ml (0–115) and TRAb: 17.84 IU/L (<1.5) were detected. US revealed normal thyroid gland sizes and highly heterogeneous parenchyma, and a minimal increase in vascularity was detected in the parenchyma with Doppler. Subsequently, increased diffuse activity uptake in both thyroid lobes was observed on thyroid scintigraphy (Fig. 1B), and GD was diagnosed. Treatment was started of propranolol and 15 mg/day MMI. Two months later, the fT4 level decreased to 0.9 ng/dL and fT3 decreased to 2.37 ng/L, and the MMI dose was reduced to 10 mg/day.
3.3. Rapidly developing Graves’ ophthalmopathy
3.3.1. Patient 5
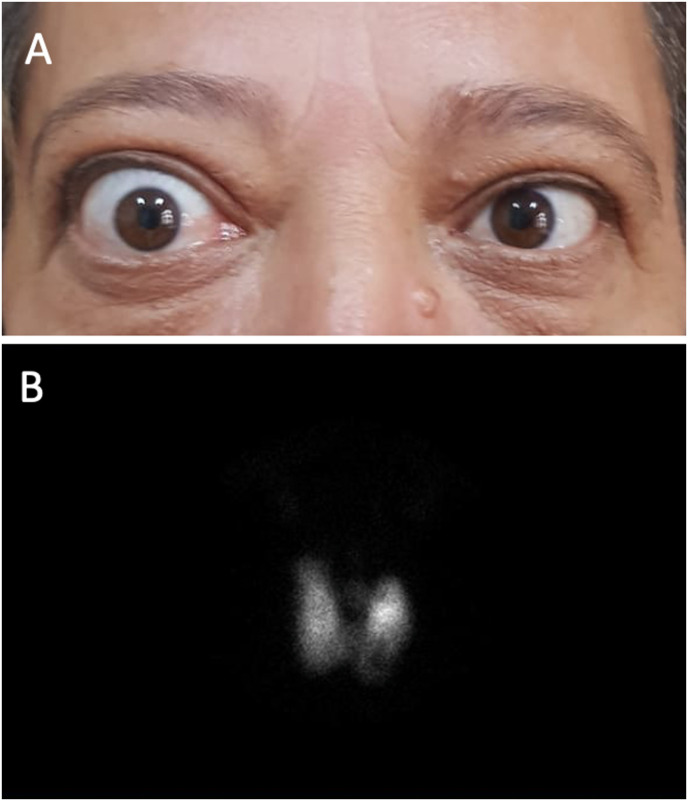
A 51-year-old female patient with no known history of thyroid disease and living in an iodine-deficient area was referred to our outpatient clinic in August 2021 due to the detection of hyperthyroidism and exophthalmos. The patient, who had diabetes and hypertension under control with medication, had impaired blood pressure control for the last 6 weeks, and complaints of palpitations, sweating, and fever. She had received the first dose of mRNA COVID-19 vaccine (Pfizer-BioNTech®) in early June 2021 and the second dose 1 month later. Complaints of proptosis, irritation, and dryness, especially in the right eye, started 4 days after the second dose and gradually increased. The patient stated that her eyes were completely normal in the period before the vaccine. On physical examination, the thyroid gland was palpated as nodular enlargement and there was mild-to-moderate active Graves’ ophthalmopathy (GO) with a clinical activity score (CAS) of 3 in the right eye (Fig. 2 A). Thyroid hormone and antibody panel results were TSH<0.01 mIU/L (0.27–4.2), fT4: 3.72 ng/dL (0.93–1.7), fT3: 12.6 ng/L (2–4.4), anti-TPO: 12.4 IU/ml (0–34), anti-Tg: 18.2 IU/ml (0–115), and TRAb: 5.04 IU/L (<1.5). On US examination, the size of the thyroid gland was greatly enlarged and there was a multinodular goiter, the largest of which was a 22 mm iso-hypoechoic nodule on the longitudinal axis in the left lower lobe. Thyroid scintigraphy showed a hypoactive multinodular hyperplasic thyroid gland in the background of hyperthyroidism (Fig. 2B). With the diagnosis of GD, 15 mg/day MMI and propranolol were started. In the 4-month follow-up, despite the hyperthyroidism being under control, the decision was made for total thyroidectomy by the multidisciplinary council in December 2021 for the patient who had progressive ocular findings, increased TRAb levels, multinodular goiter, and was willing to have surgery. Histopathological examination revealed a 12 mm classic type papillary thyroid carcinoma focus in the nodule in the left lobe, while the right lobe and isthmus were consistent with nodular hyperplasia and lymphocytic thyroiditis. After thyroidectomy, her ocular findings showed a significant regression.
Fig. 2.
(A) Patient 5: severe proptosis, eyelid retraction, mild periorbital edema, and 1+ chemosis seen in the right eye two months after the second dose of vaccine, (B) scintigraphy image of the same patient with bilaterally increased technetium pertechnetate uptake in the thyroid gland and decreased activity uptake in the left thyroid lower lobe in anterior pinhole view.
3.4. Newly diagnosed Graves’ disease
3.4.1. Patient 6
A 47-year-old female patient with no known history of thyroid disease was referred to our clinic after hyperthyroidism was detected prior to the cholecystectomy surgery. The patient was found to be euthyroid in routine control examinations in 2020. The patient had not had SARS-CoV-2 infection, and complaints of sweating and palpitations had been ongoing for 3 months. She had received the first dose of mRNA COVID-19 vaccine (Pfizer-BioNTech®) at the beginning of June 2021 and the second dose 1 month later, and the current complaints had started 5 days after the first dose of vaccination. It was revealed that her complaints continued without aggravation after the second dose of vaccination. Thyroid hormone and antibody panel results were TSH<0.01 mIU/L, fT4: 3.32 ng/dL, fT3: 11.0 ng/L, anti-TPO:11.2 IU/ml (0–34), anti-Tg: 320 IU/ml (0–115), and TRAb: 22.74 IU/L (<1.5). In addition, when laboratory tests were evaluated retrospectively, it was observed that the patient had overt hyperthyroidism 20 days after the first dose of the vaccine. On US examination, diffuse millimetric hypoechoic areas in the bilaterally enlarged thyroid gland and moderate vascularity increase in the parenchyma with Doppler were observed. With the diagnosis of GD, treatment was started of MMI 15 mg/day and propranolol. One month later, the fT4 level decreased to 1.72 ng/dL and fT3 decreased to 4.18 ng/L.
3.4.2. Patient 7
A healthy, 46-year-old male patient with no known history of thyroid disease presented with symptoms of hyperthyroidism at the beginning of October 2021. The patient had not had any recent laboratory examination, and did not have a documented SARS-CoV-2 infection. He had complaints of emotional lability, sweating, palpitations, and weight loss ongoing for 2 months. The patient had received the second dose of mRNA COVID-19 vaccine (Pfizer-BioNTech®) one month after the first dose in mid-July 2021 and the current complaints started approximately 3 weeks after the second dose of vaccination. Thyroid hormone and antibody panel results were TSH<0.01 mIU/L (0.27–4.2), fT4>7.77 ng/dL (0.93–1.7), fT3: 25.30 ng/L (2–4.4), anti-TPO: 146 IU/ml (0–34), anti-Tg: 334 IU/ml (0–115) and TRAb: 9.10 IU/L (<1.5). US showed diffuse millimetric hypoechoic areas in the bilaterally enlarged thyroid gland and increased parenchymal vascularity in a ‘Thyroid inferno’ pattern with Doppler. In addition, an isoechoic thyroid nodule with a diameter of 34 mm in the longitudinal axis was detected. MMI 20 mg/day and propranolol were started with the diagnosis of GD in the patient whose present nodule was hypoactive on thyroid scintigraphy. One month later, the fT4 level decreased to 1.97 ng/dL and fT3 decreased to 6.76 ng/L and the MMI dose was increased to 25 mg/day.
3.5. Literature review
The COVID-19 vaccine-associated GD cases published until the end of December 2021 and the current cases were summarized in Table 1 . It was identified that 13 GD cases associated with the COVID-19 vaccine have been reported [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13]]. Eight (61.5%) of these cases were women, and the median age at diagnosis was 40 (range, 28–71) years. Five of the reported cases had a previous history of AITD [[4], [5], [6], [7], [8]]. Nine patients were associated with the mRNA COVID-19 vaccine, while the remaining 4 were associated with the vectored COVID-19 vaccine. The median post-vaccination symptom onset was 10 (range, 2–38) days.
Table 1.
Reported cases of Graves’ Disease after COVID-19 vaccination.
| Author reference | Age/Sex | Disease history | Presence of COVID-19 history | COVID-19 vaccine name -dose | Pre-vaccination thyroid hormone statusa | Post-vaccination symptom onset (days) |
Laboratory test results at diagnosis |
Antithyroid treatment | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TSH (RR) | fT4 (RR) | fT3 (RR) | Anti-TPO | Anti-Tg | TRAb (RR) | ||||||||
| Relapsed Graves' Disease | |||||||||||||
| Current patient 1 | 44/F | GD in remission (for 12 years) | No | CoronaVac® - 1st | N/A | 7 | <0.01 mIU/L (0.27–4.2) | 2.67 ng/dL (0.93–1.7) | 9.65 ng/L (2–4.4) | + | + | 12.2 IU/L (<1.5) | MMI 20 mg/day |
| Current patient 2 | 49/M | GD in remission (for 1 year) | No | Pfizer-BioNTech® - 2nd | Euthyroid without treatment | 30 | <0.01 mIU/L (0.27–4.2) | 3.86 ng/dL (0.93–1.7) | 13.5 ng/L (2–4.4) | + | + | 3.01 IU/L (<1.5) | MMI 20 mg/day |
| Current patient 3 | 31/F | GD in remission (for 1 year) | No | Pfizer-BioNTech® - 1st | Euthyroid without treatment | 21 | <0.01 mIU/L (0.27–4.2) | >7.77 ng/dL (0.93–1.7) | 21.7 ng/L (2–4.4) | + | – | 19.3 IU/L (<1.5) | MMI 20 mg/day |
| [4] | 30/F | Active GD (for 3 years) | N/A | ChAdOx1 nCoV19 (Oxford-AstraZeneca) - 1st | Euthyroid with MMI 2,5 mg/day | 4 | N/A | N/A | N/A | N/A | N/A | 13.4 IU/L (0–1.75) | MMI switched to 5 mg/day |
| [5] | 34/F | GD in remission (for 11 years) | N/A | Pfizer-BioNTech® -1st | Euthyroid without treatment | 10 | <0.01 mU/L (0.4–2.75) | 2.54 ng/dL (0.75–1.6) | 22.1 pmol/L (3–6.5) | N/A | N/A | 40 IU/L (<0.55) | MMI 40 mg/day |
| [6] | 71/F | GD in remission (for 17 years) | N/A | Pfizer-BioNTech® - 2nd | N/A | 35 | N/A | 3.56 ng/dL (0.7–1.7) | 11.1 pg/mL (2.15–4.1) | N/A | N/A | 4.2 IU/L (<1.5) | N/A |
| Conversion from Hashimoto's thyroiditis to Graves' disease | |||||||||||||
| Current patient 4 | 53/F | Hashimoto's thyroiditis (for 2 years) | Yes | Pfizer-BioNTech® - 2nd | Euthyroid with LT replacement | 7 | <0.01 mIU/L (0.27–4.2) | 4.01 ng/dL (0.93–1.7) | 8.83 ng/L (2–4.4) | + | + | 17.8 IU/L (<1.5) | MMI 15 mg/day |
| [7] | 40/F | Hashimoto's thyroiditis (for 8 years) | No | Pfizer-BioNTech® -2nd | Euthyroid with LT replacement | ∼30 | 0.02 mIU/L (0.47–4.68) | 66.6 pmol/L (10–28.2) | 30.5 pmol/L (2–4.4) | + | + | 420% (<140%) | CMZ |
| Rapidly developing Graves' ophthalmopathy | |||||||||||||
| Current patient 5 | 51/F | DM, HT | No | Pfizer-BioNTech® - 2nd | Euthyroid | 4 | <0.01mIU/L (0.27–4.2) | 3.72 ng/dL (0.93–1.7) | 12.6 ng/L (2–4.4) | – | – | 5.04 IU/L (<1.5) | MMI 15 mg/day |
| [8] | 50/F | GD in remission (for 11 years) | N/A | Pfizer-BioNTech® - 2nd | Euthyroid with LT replacement | 3 | Normal | Normal | Normal | N/A | N/A | 2.29 IU/L (<0.55) | – |
| Newly diagnosed Graves' Disease | |||||||||||||
| Current patient 6 | 47/F | Obesity | No | Pfizer-BioNTech®-1st | Euthyroid | 5 | <0.01 mIU/L (0.27–4.2) | 3.32 ng/dL (0.93–1.7) | 11.0 ng/L (2–4.4) | – | + | 22.7 IU/L (<1.5) | MMI 15 mg/day |
| Current patient 7 | 46/M | – | No | Pfizer-BioNTech®-2nd | N/A | 21 | <0.01 mIU/L (0.27–4.2) | >7.77 ng/dL (0.93–1.7) | 25.3 ng/L (2–4.4) | + | + | 9.1 IU/L (<1.5) | MMI 25 mg/day |
| [6] | 46/M | – | N/A | Pfizer-BioNTech® - 1st | Euthyroid | 15 | N/A | 1.63 ng/dL (0.7–1.7) | 5.2 pg/mL (2.15–4.1) | N/A | N/A | 2.9 IU/L (<1.5) | N/A |
| [9] | 32/M | – | N/A | Vaxzevria® (Oxford-AstraZeneca) - 2nd | N/A | 10 | 0.005 uIU/mL (N/A) | 2.96 ng/dL (0.6–1.12) | 7.9 pg/mL (2–4.4) | N/A | N/A | 7.98 IU/L (<2.9) | MMI 15 mg/day |
| [9] | 35/M | – | N/A | Vaxzevria® (Oxford-AstraZeneca) - 1st | N/A | 5 | 0.004 uIU/mL (N/A) | 4.96 ng/dL (0.6–1.12) | N/A | N/A | N/A | 3.2 IU/L (<2.9) | MMI 15 mg/day |
| [10] | 52/M | Vitiligo, DM | No | Pfizer-BioNTech® - 2nd | N/A | 30 | 0.004 mIU/L (0.4–4) | 5.56 ng/dL (0.7–1.7) | 15.0 ng/L (2.7–5.7) | + | + | 6.48 IU/L (0–1.49) | MMI |
| [11] | 38/F | – | N/A | Pfizer-BioNTech® - 1st | N/A | 12 | 0.008 uIU/mL (0.35–4.95) | 2.01 ng/dL (0.7–1.48) | 7.46 pg/mL (1.58–3.9) | + | + | 12.5 IU/L (<0.7) | MMI |
| [12] | 70/M | N/A | No | ChAdOx1 nCoV19 (Oxford-AstraZeneca) – 2nd | Euthyroid | 2 | 0.003 mIU/L (0.35–4.94) | 3.19 ng/dL(0.7–1.48) | 20.0 pg/mL (2.04–4.4) | N/A | N/A | 3.23 IU/L (<1.75) | MMI 15 mg/day |
| [13] | 40/F | HT | Yes | Pfizer-BioNTech® - 1st | N/A | 2 | <0.001 mIU/L (0.27–4.4) | 3.57 ng/dL (0.93–1.71) | 10.5 pg/mL (2.04–4.4) | + | + | 16.6 IU/L (<1.75) | N/A |
| [13] | 28/F | – | No | Pfizer-BioNTech® - 1st | N/A | 3 | <0.001 mIU/L (0.27–4.4) | 1.84 ng/dL (0.93–1.71) | 9.2 pg/mL (2.04–4.4) | + | + | 5.85 IU/L (<1.75) | N/A |
COVID-19: Coronavirus disease-2019; GD: Graves' disease; DM: Diabetes Mellitus; HT: Hypertension; TSH: thyroid-stimulating hormone; fT4: free thyroxine; fT3: free triiodothyronine; Anti-TPO: anti-thyroid peroxidase; Anti-Tg: anti-thyroglobuline; TRAb: TSH receptor antibody; MMI: methimazole; CMZ: carbimazole; LT: levothyroxine; N/A: not available.
Indicates the thyroid hormone status in the period 6 months before vaccination.
4. Discussion
In this paper, 7 GD patients consisting of relapsed and newly diagnosed cases following the COVID-19 vaccination were presented with four different onset histories. Five of the current patients (71.4%) were female and the median age of the patients was 47 years (range, 31–53). In the current study, the first case of GD associated with an inactivated COVID-19 vaccine was presented, while other cases were associated with the mRNA COVID-19 vaccine. The median post-vaccination symptom onset was 7 days (range, 4–30). Considered together with the review of literature, it seems that COVID-19 vaccine-associated GD cases are similar to classical GD cases in terms of age and gender, and symptoms begin approximately 7–10 days after vaccination (Table 1). It was noteworthy that approximately half of the patients had a previous history of AITD (4 patients in the current presentation, 5 patients in the literature) (Table 1) [[4], [5], [6], [7], [8]]. In addition, as in cases of classical GD, the majority of patients with vaccine-associated GD had increased titers of anti-TPO and anti-Tg titers, as well as TRAb [15].
During the pandemic, it has been well observed that SARS-CoV-2 causes damage to many tissues and organs by causing a hyperactive immune response in the individual. This is also true for thyroid follicular cells that express the ACE-2 receptor that SARS-CoV-2 uses to enter the cells [16]. There are reports in the literature of cases of GD, Hashimoto's thyroiditis, and subacute thyroiditis developing in the post-COVID-19 period [3]. Likewise, some autoimmune/inflammatory thyroiditis cases thought to be associated with immunization against COVID-19 are still being reported, with the first GD cases reported in May 2021 [13]. It has been argued that the potential cause may be inappropriate reactogenicity triggered by the adjuvants used to increase the immunogenicity of the vaccine [13,17]. This phenomenon, also known as autoimmune/inflammatory syndrome induced by adjuvants (ASIA), was first described by Shoenfeld and Agmon-Levin in 2011 [18]. In the present study, the inactivated COVID-19 vaccine, which is shown in this paper for the first time to be associated with GD, also contains aluminum hydroxide as an adjuvant, and this adjuvant may have contributed to the formation of the hyperimmune response in the thyroid gland by activating various immune cascades.
Three-quarters of the cases identified to date have been associated with the COVID-19 mRNA vaccine, which contains no known adjuvants. However, recently, Alameh et al. demonstrated that the nucleoside-modified mRNA encapsulated in lipid nanoparticles (mRNA-LNPs) included in the Pfizer-BioNTech® COVID-19 vaccine strongly affected the T follicular helper cell and humoral responses, leading to immunostimulation [19]. This suggests that mRNA-LNPs may themselves act as an adjuvant and lead to ASIA syndrome. On the other hand, Vojdani et al. determined that SARS-CoV-2 proteins (spike protein, membrane protein, and nucleoprotein) have a significant number of amino acid sequence similarities with TPO proteins ranging from 50 to 70% [20]. In addition, Kanduc and Shoenfeld also demonstrated that the SARS-CoV-2 spike proteins are molecularly similar mainly to human, mouse, and rat hexa/heptapeptides, at a much higher rate compared to other coronavirus family members [21]. In the light of these studies, both the cross-reactivity resulting from the molecular mimicry of SARS-CoV-2 spike proteins with TSH receptor antigens and/or other thyroid proteins, and the evidence that LNPs act as adjuvants and stimulate a robust immune response suggest that multiple mechanisms may be involved in the occurrence of GD cases following the COVID-19 mRNA vaccination.
In the current study, three patients had relapsed GD after vaccination. Relapse develops in approximately 50% of patients in GD, and recent reviews have shown that two-thirds of these relapses occur 6–18 months after stopping antithyroid treatment [22]. According to 2016 American Thyroid Association guidelines, patients who are still euthyroid 12 months after drug discontinuation are considered in remission [23]. While relapse after years in patient 1 suggests the possible causal relationship, relapse of the disease one year after drug discontinuation in patients 2 and 3 can also be considered as a natural course of GD. However, the fact that both cases, which were known to be euthyroid shortly before the vaccine, became symptomatic in the post-vaccine period, make it necessary to question a possible interaction. In addition, cases in the literature of GD that relapsed after COVID-19 vaccine, two after 11 years and one after 17 years, strengthen the possible relationship [5,6,8]. Moreover, in a case with active GD [4], the increase in the antithyroid dose requirement after COVID-19 vaccination also indicates that vaccination may aggravate the pre-existing autoimmune disease. Although the factors causing relapse are still not clearly known, the current cases warn that vaccination may be a trigger factor for GD relapse.
Conversion from Hashimoto's thyroiditis to GD has been reported with limited case series [24]. In these patients, some factors affecting the immune response (e.g. drug replacement therapy, pregnancy) are thought to trigger the conversion of thyroid blocking antibodies to stimulating antibodies [25]. In addition to the case reported in this paper, Lui et al. also described a case with a history of hypothyroidism who developed COVID-19 mRNA vaccine-associated GD [7]. Although not addressed to date, the current cases demonstrate that vaccination may induce antibody conversion in hypothyroid individuals receiving LT replacement.
Although half of GD patients have ocular symptoms, 3–5% of GD patients develop severe GO [26]. Ocular symptoms often appear with the onset of hyperthyroidism or within 18 months [26]. Interestingly, in patient 5, we were faced with simultaneously developing GD and unilateral GO and coincidental papillary thyroid carcinoma after the COVID-19 mRNA vaccination. This patient, who was living in an iodine-deficient area, had a previously undetected multinodular goiter. Recently, Rubinstein et al. described a thyroid eye disease that developed rapidly after the COVID-19 mRNA vaccine and was controlled with teprotumumab in a patient diagnosed with GD 11 years previously and who was being followed up in remission [8]. These cases suggest that GO as well as GD may develop more rapidly than normal in the hyperimmune environment created after the COVID-19 immunization.
In conclusion, vaccines carrying inactivated particles or mRNA of SARS-CoV-2 may make the thyroid gland a potential target by the aforementioned hypothetical mechanisms. The fact that most cases of vaccine-associated GD have been reported after mRNA vaccination highlights the possibility of immune cross-reactivity as well as the adjuvant effects of LNPs. In patients with a previous history of AITD (especially GDs in remission), where an autoimmune response can be triggered more easily, attention should be paid to relapse in the post-vaccination period. With these few reported cases, it would not be correct to make definitive judgments about the relationship between the COVID-19 vaccine and GD, and the vaccination program must be followed in order to control the pandemic. In this regard, multicenter, prospective studies are needed to explain the causal relationship and better elucidate the pathogenetic mechanisms.
Disclosure
No financial grant has been received for the current study. Written informed consent was obtained from all patients for publication of this study and accompanying images.
Author statements
Hayri Bostan: Conseptualization, Investigation, Data curation, Writing – original draft. Bekir Ucan: Conseptualization, Investigation, Writing – original draft. Muhammed Kizilgul: Writing – review & editing, Supervision. Murat Calapkulu: Investigation, Data curation. Sema Hepsen: Writing – review & editing. Umran Gul: Data curation. Ilknur Ozturk Unsal: Writing – review & editing. Erman Cakal: Writing – review & editing, Supervision.
Declaration of competing interest
The authors have no conflicts of interest to disclose.
References
- 1.Davies T.F., Andersen S., Latif R., Nagayama Y., Barbesino G., Brito M., et al. Graves' disease. Nat. Rev. Dis. Prim. 2020;6:52. doi: 10.1038/s41572-020-0184-y. https://doi:10.1038/s41572-020-0184-y [DOI] [PubMed] [Google Scholar]
- 2.Oldstone M.B.A. Molecular mimicry: its evolution from concept to mechanism as a cause of autoimmune diseases. Monoclon. Antibodies Immunodiagn. Immunother. 2014;33:158–165. doi: 10.1089/mab.2013.0090. https://doi:10.1089/mab.2013.0090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murugan A.K., Alzahrani A.S. SARS-CoV-2: emerging role in the pathogenesis of various thyroid diseases. J. Inflamm. Res. 2021;14:6191–6221. doi: 10.2147/JIR.S332705. https://doi:10.2147/JIR.S332705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sriphrapradang C. Aggravation of hyperthyroidism after heterologous prime-boost immunization with inactivated and adenovirus-vectored SARS-CoV-2 vaccine in a patient with Graves' disease. Endocrine. 2021;74:226–227. doi: 10.1007/s12020-021-02879-8. https://doi:10.1007/s12020-021-02879-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pierman G., Delgrange E., Jonas C. Recurrence of Graves' disease (a Th1-type cytokine disease) following SARS-CoV-2 mRNA vaccine administration: a simple coincidence? Eur. J. Case Rep. Intern. Med. 2021;8 doi: 10.12890/2021_002807. https://doi:10.12890/2021_002807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zettinig G., Krebs M. Two further cases of Graves' disease following SARS-Cov-2 vaccination. J. Endocrinol. Invest. 2021 doi: 10.1007/s40618-021-01650-0. https://doi:10.1007/s40618-021-01650-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lui D.T.W., Lee K.K., Lee C.H., Lee A.C.H., Hung I.F.N., Tan K.C.B. Development of Graves' disease after SARS-CoV-2 mRNA vaccination: a case report and literature review. Front. Public Health. 2021;9:778964. doi: 10.3389/fpubh.2021.778964. https://doi:10.3389/fpubh.2021.778964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubinstein T.J. Thyroid eye disease following COVID-19 vaccine in a patient with a history Graves' disease: a case report. Ophthalmic Plast. Reconstr. Surg. 2021;37:e221–e223. doi: 10.1097/IOP.0000000000002059. https://doi:10.1097/IOP.0000000000002059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.di Filippo L., Castellino L., Giustina A. Occurrence and response to treatment of Graves' disease after COVID vaccination in two male patients. Endocrine. 2021 doi: 10.1007/s12020-021-02919-3. https://doi:10.1007/s12020-021-02919-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patrizio A., Ferrari S.M., Antonelli A., Fallahi P. A case of Graves' disease and type 1 diabetes mellitus following SARS-CoV-2 vaccination. J. Autoimmun. 2021;125:102738. doi: 10.1016/j.jaut.2021.102738. https://doi:10.1016/j.jaut.2021.102738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pujol A., Gómez L.-A., Gallegos C., Nicolau J., Sanchís P., González-Freire M., et al. Thyroid as a target of adjuvant autoimmunity/inflammatory syndrome due to mRNA-based SARS-CoV2 vaccination: from Graves' disease to silent thyroiditis. J. Endocrinol. Invest. 2021 doi: 10.1007/s40618-021-01707-0. https://doi:10.1007/s40618-021-01707-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sriphrapradang C., Ch Shantavasinkul P. Graves' disease following SARS-CoV-2 vaccination. Endocrine. 2021;74:473–474. doi: 10.1007/s12020-021-02902-y. https://doi:10.1007/s12020-021-02902-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vera-Lastra O., Navarro A.O., Domiguez M.P.C., Medina G., Valadez T.I.S., Jara L.J. Two cases of Graves' disease following SARS-CoV-2 vaccination: an autoimmune/inflammatory syndrome induced by adjuvants. Thyroid. 2021;31:1436–1439. doi: 10.1089/thy.2021.0142. https://doi:10.1089/thy.2021.0142 [DOI] [PubMed] [Google Scholar]
- 14.Leiva S., Madrazo J., Podesta C. Narcolepsy with cataplexy and hyperthyroidism sudden appeared after H1N1 vaccination. Sleep Sci. 2018;11:34–36. doi: 10.5935/1984-0063.20180008. https://doi:10.5935/1984-0063.20180008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fröhlich E., Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front. Immunol. 2017;8:521. doi: 10.3389/fimmu.2017.00521. https://doi:10.3389/fimmu.2017.00521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazartigues E., Qadir M.M.F., Mauvais-Jarvis F. Endocrine significance of SARS-CoV-2’s reliance on ACE2. Endocrinology. 2020;161 doi: 10.1210/endocr/bqaa108. https://doi:10.1210/endocr/bqaa108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bragazzi N.L., Hejly A., Watad A., Adawi M., Amital H., Shoenfeld Y. ASIA syndrome and endocrine autoimmune disorders. Best Pract. Res. Clin. Endocrinol. Metabol. 2020;34:101412. doi: 10.1016/j.beem.2020.101412. https://doi:10.1016/j.beem.2020.101412 [DOI] [PubMed] [Google Scholar]
- 18.Shoenfeld Y., Agmon-Levin N. ‘ASIA’–autoimmune/inflammatory syndrome induced by adjuvants. J. Autoimmun. 2011;36:4–8. doi: 10.1016/j.jaut.2010.07.003. https://doi:10.1016/j.jaut.2010.07.003 [DOI] [PubMed] [Google Scholar]
- 19.Alameh M.-G., Tombacz I., Bettini E., Lederer K., Sittplangkoon C., Wilmore J.R., et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity. 2021;54:2877–2892. doi: 10.1016/j.immuni.2021.11.001. https://doi:10.1016/j.immuni.2021.11.001 e1-e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vojdani A., Vojdani E., Kharrazian D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: implications for autoimmune diseases. Front. Immunol. 2021;11:617089. doi: 10.3389/fimmu.2020.617089. https://doi:10.3389/fimmu.2020.617089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanduc D., Shoenfeld Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunol. Res. 2020;68:310–313. doi: 10.1007/s12026-020-09152-6. https://doi:10.1007/s12026-020-09152-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Struja T., Fehlberg H., Kutz A., Guebelin L., Degen C., Mueller B., et al. Can we predict relapse in Graves' disease? Results from a systematic review and meta-analysis. Eur. J. Endocrinol. 2017;176:87–97. doi: 10.1530/EJE-16-0725. https://doi:10.1530/EJE-16-0725 [DOI] [PubMed] [Google Scholar]
- 23.Ross D.S., Burch H.B., Cooper D.S., Greenlee M.C., Laurberg P., Maia A.L., et al. American tyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of tyrotoxicosis. Thyroid. 2016;26:1343–1421. doi: 10.1089/thy.2016.0229. https://doi:10.1089/thy.2016.0229 2016. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Aguilera B., Betea D., Lutteri L., Cavalier E., Geenen V., Beckers A., et al. Conversion to Graves disease from Hashimoto thyroiditis: a study of 24 patients. Arch. Endocrinol. Metab. 2018;62:609–614. doi: 10.20945/2359-3997000000086. https://doi:10.20945/2359-3997000000086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLachlan S.M., Rapoport B. Thyrotropin-blocking autoantibodies and thyroid-stimulating autoantibodies: potential mechanisms involved in the pendulum swinging from hypothyroidism to hyperthyroidism or vice versa. Thyroid. 2013;23:14–24. doi: 10.1089/thy.2012.0374. https://doi:10.1089/thy.2012.0374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahn R.S. Graves' ophthalmopathy. N. Engl. J. Med. 2010;362:726–738. doi: 10.1056/NEJMra0905750. https://doi:10.1056/NEJMra0905750 [DOI] [PMC free article] [PubMed] [Google Scholar]