Key Points
Question
What is the burden of adrenalectomy in the context of multivisceral resection?
Findings
In this observational study that included 50 adults receiving surgery for retroperitoneal sarcoma, the incidence of adrenal insufficiency was 64% in the early postoperative period and 38.5% at longer follow-up.
Meaning
In this study, adrenal insufficiency after multivisceral resection with en bloc adrenalectomy was frequent when specifically investigated, so patients at risk should be monitored to exclude underrated impairment of adrenal function.
This observational study evaluates postoperative adrenal insufficiency in adult patients undergoing multivisceral resection, including en bloc adrenalectomy.
Abstract
Importance
The risk of developing adrenal insufficiency (AI) following adrenalectomy has been insufficiently studied in the context of multivisceral resection (MVR).
Objective
To evaluate the incidence of AI in patients undergoing MVR with en bloc adrenalectomy.
Design, Setting, and Participants
Prospective observational longitudinal study in a single referral center including 56 consecutive adult patients undergoing retroperitoneal sarcoma surgery from June 2019 to August 2020. Those who were candidates for MVR with en bloc adrenalectomy and had no preexisting adrenal impairment were considered eligible. Of these, 4 individuals were excluded because they did not receive adrenalectomy at the time of surgery and 2 because they were not considered evaluable for the main end point. Follow-up was set at 4 months after surgery, and 49 patients completed follow-up. Data were analyzed from October 2020 to September 2021.
Exposures
Diagnosis of AI was determined by low-dose (1 μg) adrenocorticotropic hormone (ACTH) stimulation test with a threshold of 20 μg/dL in blood samples retrieved 30 and 60 minutes after stimulation. ACTH test was repeated on postoperative days 1 and 10 and at 4 months’ follow-up.
Main Outcome and Measures
The primary end point was incidence and relevance of AI after MVR. Secondary end points were associations with patient- and tumor-related factors, impact on perioperative hemodynamic management, and association with postoperative morbidity and mortality.
Results
Fifty patients (26 female; median [IQR] age, 59 [46-67] years) were evaluable. Incidence of AI was 64% (32 of 50 patients) in the early postoperative period and 38.5% (15 of 39 patients) at follow-up. Patients with AI showed lower postoperative cortisol values. Factors associated with risk of AI at univariate analysis were high American Society of Anesthesiologists score (odds ratio [OR], 0.31; 95% CI, 0.14-0.48) and high malignancy grade (OR, 0.35; 95% CI, 0.24-0.46). Clinical outcomes not associated with AI included morbidity, mortality, reoperation rate, admission to intensive care unit, length of intensive care unit stay, total hospital stay, and long-term quality of life.
Conclusions and Relevance
In this study, AI after MVR with en bloc adrenalectomy was frequent, even in patients with adequate preoperative adrenal function. Despite this, adrenalectomy can be safely performed. Patients at risk should be monitored in the long term to exclude underrated impairment of adrenal function.
Introduction
Resection of a healthy adrenal gland and its relevance in postoperative recover and quality of life are poorly studied. In general surgery, indication to adrenalectomy in the context of a multivisceral resection (MVR) lacks robust data regarding outcomes and adverse effects. Surgery for retroperitoneal sarcoma (RPS) is an ideal study model for addressing these deficiencies. Surgical treatment for RPS often requires MVR en bloc with the retroperitoneal tumor.1,2,3,4 This surgical strategy implies the need for ipsilateral adrenalectomy in roughly 50% of all RPS cases, as the extension of resection is driven by the histopathological characteristic and anatomic extent of the disease rather than the actual infiltration of the adrenal gland.5,6,7
We hypothesized that en bloc resection of a healthy adrenal gland may impact the postoperative course. This study aimed at investigating postoperative adrenal function in a prospective series of patients affected by primary RPS undergoing operation at our institution.
Methods
Study Design and Participants
This was a prospective observational longitudinal single-center study. All patients provided written informed consent. The study was approved by the institutional review board at Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Patients candidate to MVR were screened for the following eligibility criteria: 18 years and older, proven diagnosis of primary RPS, and en bloc adrenalectomy indicated based on preoperative imaging. Exclusion criteria included clinically relevant adrenal impairment, defined as primary adrenal gland disease or preoperative basal cortisol level less than 7 μg/dL; chronic steroid therapy; clinically significant heart disease or thyroid dysfunction; and local recurrence of sarcoma or presence of distant metastasis. Given logistic capacity, enrollment was limited to no more than 3 patients at a time.
From June 2019 to August 2020, 116 patients underwent surgery for primary RPS at our center, 67 of whom did not meet eligibility criteria and were excluded. Among 56 patients enrolled in the study, 4 patients did not undergo adrenalectomy based on intraoperative reassessment. Owing to intercurrent clinical conditions, 2 patients did not receive adrenocorticotropic hormone (ACTH) test on postoperative day 10 and were therefore excluded from the analysis (eFigure 1 in the Supplement). The final study population included 50 evaluable patients. Detailed clinical and pathological features are reported in Table 1.
Table 1. Overall Study Population and General Characteristics According to Occurrence of Adrenal Insufficiency (AI).
| Characteristic | No. (%) | P value | ||
|---|---|---|---|---|
| Overall population | Postoperative AI | |||
| Yes | No | |||
| No. of patients | 50 | 32 (64) | 18 (32) | NA |
| Age, median (IQR), y | 59 (46-67) | 61 (43-67) | 56 (51-68) | .98 |
| Female | 24 | 16 | 8 | .77 |
| Male | 26 | 16 | 10 | |
| BMI, median (IQR) | 25.4 (23.2-27.4) | 25.1 (22.7-27.6) | 25.7 (23.4-27.3) | .87 |
| ASA score | <.05 | |||
| 1 | 1 (2) | 1 (3.1) | 0 | |
| 2 | 38 (76) | 21 (65.6) | 17 (94.4) | |
| 3 | 11 (22) | 10 (31.3) | 1 (5.6) | |
| AKI score | .70 | |||
| 1 | 27 (54) | 18 (56.3) | 9 (50) | |
| 2 | 17 (24) | 10 (31.3) | 7 (38.8) | |
| 3 | 4 (8) | 2 (6.2) | 2 (11.2) | |
| 4 | 2 (4) | 2 (6.2) | 0 | |
| Histology | .83 | |||
| WDLPS | 5 (10) | 2 (6.2) | 3 (16.6) | |
| DDLPS | 32 (64) | 18 (56.3) | 14 (77.8) | |
| LMS | 8 (16) | 7 (21.9) | 1 (5.6) | |
| Other | 5 (10) | 5 (15.6) | 0 | |
| FNCLCC grade | .02 | |||
| 1 | 7 (14) | 4 (12.4) | 3 (16.7) | |
| 2 | 33 (66) | 18 (56.3) | 15 (83.3) | |
| 3 | 10 (20) | 10 (31.3) | 0 | |
| Adrenal invasiona | .02 | |||
| No invasion | 34 (70.8) | 25 (80.6) | 9 (52.9) | |
| Perivisceral | 14 (29.2) | 6 (19.4) | 8 (47.1) | |
| Infiltration | 0 | 0 | 0 | |
| Tumor size, median (IQR), cm | 24 (13-35) | 19 (13-32) | 28 (20-37) | .15 |
| Tumor side (left/right) | 24/26 | 15/17 | 9/9 | .83 |
| Preoperative RT | 15 (30) | 10 (31.3) | 5 (27.7) | .80 |
| Preoperative CT | 15 (30) | 11 (34.4) | 4 (22.2) | .36 |
| Preoperative and postoperative CT | 2 (4) | 2 (6.2) | 0 | .36 |
Abbreviations: AKI, acute kidney injury; ASA, American Society of Anesthesiologists; BMI, body mass index; CT, chemotherapy; DDLPS, dedifferentiated liposarcoma; FNCLCC, French Fédération Nationale des Centres de Lutte Contre le Cancer; LMS, leiomyosarcoma; NA, not applicable; RT, radiation therapy; WDLPS, well-differentiated liposarcoma.
Data available in 48 patients; 2 patients were missing information on final pathological report.
Procedures
Surgical treatment consisted in MVR as previously described.1 All cases were discussed within a local multidisciplinary tumor board to establish the optimal tailored treatment strategy for neoadjuvant chemotherapy or radiation therapy. Extent of organ resection was established based on histology, biopsy, preoperative treatment, if any, and preoperative images.4 A preestablished standard algorithm for goal-directed administration of fluids and vasoactive drugs was followed during both intraoperative and postoperative course to minimize confounding factors on hemodynamic parameters (eFigure 2 in the Supplement).
Outcomes
The primary outcome was the incidence of adrenal insufficiency (AI) in the early postoperative period. AI was defined as a positive ACTH stimulation test result on postoperative day 1 or 10. Test threshold was established as peak cortisol values 20 μg/dL or less sampled 30 and 60 minutes after low-dose ACTH stimulation test (1 μg corticotrophin infusion).8,9 Basal cortisol levels were also collected preoperatively and on postoperative days 1, 2, 3, and 10.
Secondary outcomes were association between AI and clinical or pathological features, impact on perioperative hemodynamic, association with morbidity and mortality, incidence of AI at 4 months’ follow-up, and quality of life. For this aim, ACTH stimulation test was repeated at least 4 months after surgery, and patients were evaluated with the Health-related Quality of Life in Addison’s Disease (AddiQoL) scale.10 In case of persistent AI, even subclinical, the patient was referred to an endocrinology team to evaluate the need for a steroid replacement therapy.
Data Collection and Statistical Analysis
Demographic characteristics and pathologic and treatment-related data were collected. A comprehensive data set of clinical parameters was gathered, and the following invasive parameters were retrieved intraoperatively and during intensive care unit (ICU) admission by means of the HemoSphere hemodynamic monitoring system (Edwards Lifesciences)11: cardiac index, stroke volume index (SVI), stroke volume variation, pulse pressure variation, mean arterial pressure (MAP); vasoactive-inotropic score (VIS) summarized the dose and potency of vasopressor therapy at each time point by mean of a single value12 and was recorded hourly during surgery and every 8 hours thereafter until withdrawal (eTable 2 in the Supplement); blood pressure, heart rate, central vein pressure, urine output, new early warning score, central venous oxygen saturation, serum lactate, arterial pH, and base excess, retrieved every 8 hours until postoperative day 3 and daily until postoperative day 10; and overall infused fluids volumes (crystalloid and colloids) and transfusions of any blood product (packed red blood cells, fresh frozen plasma, albumin), recorded during surgery and until postoperative day 10. All infusions are reported and analyzed as milliliters per kilogram per hour to normalize variations in patient weight and operative time. Prophylaxis of postoperative nausea and vomiting, corticosteroids drugs, pain control, and use of peridural catheter or patient-controlled analgesia were recorded until postoperative day 10. Hypotension was defined as MAP less than 65 mm Hg for at least 1 minute. Similarly, an SVI less than 35 mL/m2 for at least 1 minute was considered an index of low-flow state. The Hemosphere monitor recorded hemodynamic parameters every 20 seconds during surgery. Data were exported as an electronic data set for computing time-weighted average of MAP and of SVI. Time-weighted average of MAP and SVI is the calculation of the depth of MAP or SVI under the threshold values (65 mm Hg and 35 mL/m2) multiplied by the time spent in hypotension or low-flow state in minutes divided by the total duration of operation. This approach allows comparison of hemodynamic impairment between different procedures.
The number of resected organs en bloc with tumor was computed according to the RPS weighted resected organ score.13 Postoperative complications were classified according to Clavien-Dindo classification14 and described at 30 days after surgery. Mortality was described at 90 days after surgery. Quality of life was investigated with the AddiQoL questionnaire, a proven tool in patients affected by primary AI. The questionnaire investigates general health, performance status, fatigue, and sexual and work life.10
A sample size of 50 patients was needed to obtain an accuracy in AI incidence estimate (half width of the 95% CI) of no more than 14%. The distribution of patient and tumor characteristics was described using conventional statistics, such as mean or median values and IQRs for continuous variables and absolute and relative frequency for discrete variables.
Statistical comparisons of the distributions of continuous or discrete variables were based on Mann-Whitney U test and Pearson χ2 or Fisher exact test, respectively, as appropriate. Significant associations for discrete variables were summarized by means of Pearson contingency coefficient and corresponding 95% CIs. Analyses focused primarily on determining which factors were associated with AI. Given the small size of the study, joint exploration in a multivariable setting was deemed impractical. The software used for analysis was SAS version 9.4 (SAS Institute). All tests were 2-sided, and significance was set at P < .05. Follow-up was closed on April 30, 2021, and analysis started in October 2020 for early results and concluded on September 30, 2021, for follow up results.
Results
Population Analysis
Of 50 included patients, 26 were female. The median (IQR) age was 59 (46-67) years. Mean (IQR) operative time was 453 (380-515) minutes, and the median (IQR) number of resected organs was 5 (3-6). Median (IQR) intraoperative amount of crystalloid, colloid, albumin, RBC, and fresh frozen plasma were 8.19 (6.71-9.66) mL/kg/h, 1.19 (0.29-1.58) mL/kg/h, 0.03 (0-0.07) g/kg/h, 0.71 ( 0-1.35) mL/kg/h, and 0.18 (0-0) mL/kg/h, respectively. Median (IQR) intraoperative VIS was 76.5 (46.0-142.0). Intensive care unit admission was required in 32 of 50 patients (64%) with a mean (IQR) stay of 1 day (1-2), while mean (IQR) length of hospital stay was 14 (10-38) days. According to the intention-to-treat analysis including 52 patients, postoperative mortality was 0%. Postoperative morbidity of Clavien-Dindo grade 3 or higher occurred in 13 patients (23%), while reoperation rate was 10% (Table 2).
Table 2. Surgery and Intraoperative Parameters.
| Parameter | Mean (IQR) | P value | ||
|---|---|---|---|---|
| Overall population | Postoperative AI | |||
| Yes | No | |||
| No. of patients | 50 | 32 | 18 | NA |
| Anesthesia, no. (%) | ||||
| Balanced-blended | 46 (92.0) | 29 (90.6) | 17 (94.4) | >.99 |
| TIVA | 4 (8.0) | 3 (9.4) | 1 (5.6) | |
| Operative time, min | 453 (380-515) | 436 (374-519) | 459 (431-512) | .37 |
| Time from incision to ligation of adrenal vessels, min | 240 (174-299) | 237 (172-288) | 246 (176-313) | .69 |
| Time from ligation of adrenal vessels to end of surgery, min | 247 (175-253) | 257 (170-248) | 229 (189-253) | .57 |
| No. of resected organs according to the weighted organs resection score, median (IQR) | 5 (3-6) | 4 (3-5) | 6 (5-6) | .04 |
| Crystalloids, median (IQR), mL/kg/h | 8.19 (6.71-9.66) | 8.51 (7.13-9.67) | 7.78 (6.57-8.87) | .23 |
| Colloids, median (IQR), mL/kg/h | 1.19 (0.29-1.58) | 1.24 (0.55-1.55) | 0.89 (0-1.53) | .25 |
| RBC, mL/kg/h | 0.71 (0-1.35) | 0.76 (0-1.34) | 0.20 (0-1.13) | .97 |
| FFP, mL/kg/h | 0.18 (0-0) | 0.20 (0-0) | 0.14 (0-0) | .66 |
| Albumin, g/kg/h | 0.03 (0-0.07) | 0.03 (0-0.06) | 0.04 (0-0.07) | .49 |
| Total intraoperative infused volume, mL/kg/h | 10.36 (8.11-11.64) | 10.46 (9.15-11.89) | 9 (7.66-10.31) | .64 |
| Total VIS, median (IQR) | 76.5 (46.0-142.0) | 62.0 (46.0-118.8) | 122.0 (50.5-159.8) | .24 |
| VIS/h | 10.86 (5.73-17.56) | 8.97 (5.73-15.56) | 13.80 (7.60-18.50) | .30 |
| TWA-MAP <65 mm Hg | 0.99 (0.42-1.49) | 0.74 (0.36-1.24) | 1.10 (0.72-1.69) | .10 |
| TWA-SVI <35 mL/m2 | 0.98 (0.26-1.51) | 0.90 (0.18-1.44) | 1.08 (0.30-1.51) | .92 |
| Urine output, median (IQR), mL/kg/h | 0.72 (0.53-1.06) | 0.74 (0.55-1.42) | 0.67 (0.53-0.92) | .29 |
| ICU admission, no. (%) | 32 (64.0) | 21 (65.6) | 11 (61.1) | .77 |
| ICU stay, mean (range), d | 1 (1-2) | 1 (1-2) | 1 (1-2) | .83 |
| Hospital stay, d | 14 (10-19) | 14 (10-18) | 14 (11-20) | .69 |
| Complications CD grade ≥3, no. (%) | 13 (23) | 7 (21.9) | 6 (33.3) | .64 |
Abbreviations: CD, Clavien-Dindo; FFP, fresh frozen plasma; ICU, intensive care unit; MAP, mean arteriosus pressure; NA, not applicable; RBC, red blood cells; SVI, stroke volume index; TIVA, totally intravenous anesthesia; TWA, time-weighted average; VIS, Vasoactive Inotropic Score.
Primary Outcome
On postoperative day 1, ACTH stimulation test was positive in 26 of 50 patients (52%), with 11 of 26 (42.3%) having persistently positive results on postoperative day 10. Among 24 of 50 patients (48%) with negative ACTH test results on postoperative day 1, 6 (12%) showed AI with positive test results on postoperative day 10. Therefore, as per protocol, overall incidence of postoperative AI was 64% (32 of 50 patients) according to ACTH test.
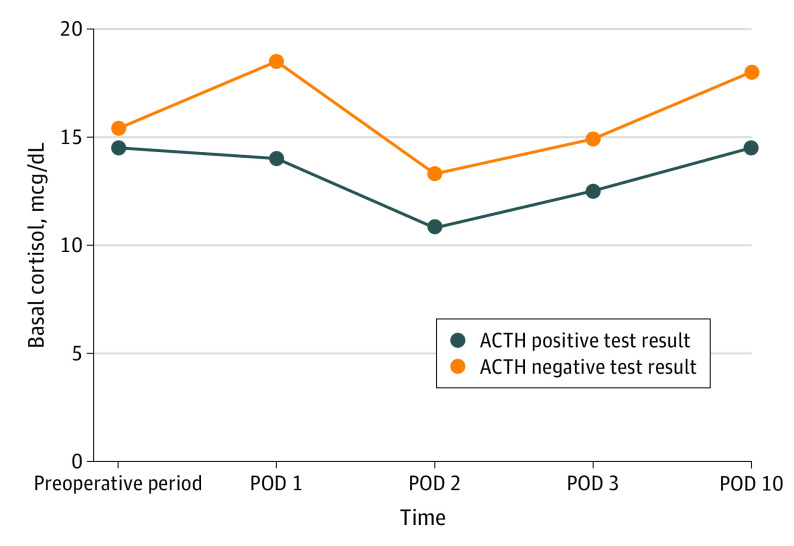
Postoperative basal cortisol levels significantly differed according to the occurrence of AI. Patients with normal adrenal function showed increased median (IQR) basal cortisol values on postoperative day 1 compared with preoperative values: 18.5 (17.0-22.0) μg/dL on postoperative day 1 vs 15.4 (11.7-18.4) μg/dL preoperatively. Conversely, patients with AI had lower median (IQR) basal cortisol values after surgery: 14.0 (10.0-18.0) μg/dL on postoperative day 1 vs 14.5 (11.6-18.5) μg/dL preoperatively. This difference was found to be statistically significant between the 2 groups of patients (P = .002). Moreover, the significant difference in basal cortisol values was maintained throughout the postoperative period, with lower median (IQR) values in patients with AI until hospital discharge (postoperative day 2 AI, 10.8 [6.4-15.5] mcg/dL vs non-AI, 13.3 [11.3-17.5] mcg/dL; P = .04; postoperative day 3 AI, 12.5 [7.8-14.7] mcg/dL vs non-AI, 14.9 [11.9-16.6] mcg/dL; P = .04; postoperative day 10 AI, 14.5 [11.5-17.0] mcg/dL vs non-AI, 18.0 [15.0-23.0] mcg/dL; P = .008) (Figure 1).
Figure 1. Basal Cortisol in the Postoperative Period.
ACTH indicates adrenocorticotropic hormone; POD, postoperative day
At univariable analysis, patients who experienced AI did not differ for age, sex, or body mass index compared with those who had normal adrenal function after surgery. Also, tumor histological subtype, median size, and preoperative treatments were similar between these 2 groups. Patients with postoperative AI had significantly higher ASA scores than those without (ASA-1, 1 patient with AI [3.1%] vs 0 patients without AI [0%]; ASA-2, 21 patients with AI [65.5%] vs 17 patients without AI [94.4%]; ASA-3, 10 patients with AI [31.3%] vs 1 patient without AI [5.4%]; P = .0497), higher tumor grade (FNCLCC-1, 4 patients with AI [12.5%] vs 3 patients without AI [16.7%]; FNCLCC-2, 18 patients with AI [56.3%] vs 15 patients without AI [83.3%]; FNCLCC-3, 10 patients with AI [31.3%] vs 0 patients without AI [0%]; P = .02), and a lower median (IQR) number of organs resected (4 [3-5] vs 6, [5-6]; P = .04). Analysis of adrenal gland on final pathology did not demonstrate parenchymal invasion in any specimen, while microscopic perivisceral invasion was found in 14 of 48 individuals (30%), significantly higher in the group of patients with normal postoperative adrenal function (9 of 18 [50.0%] vs 6 of 32 [18.8%]; P = .02) (Tables 1 and 2).
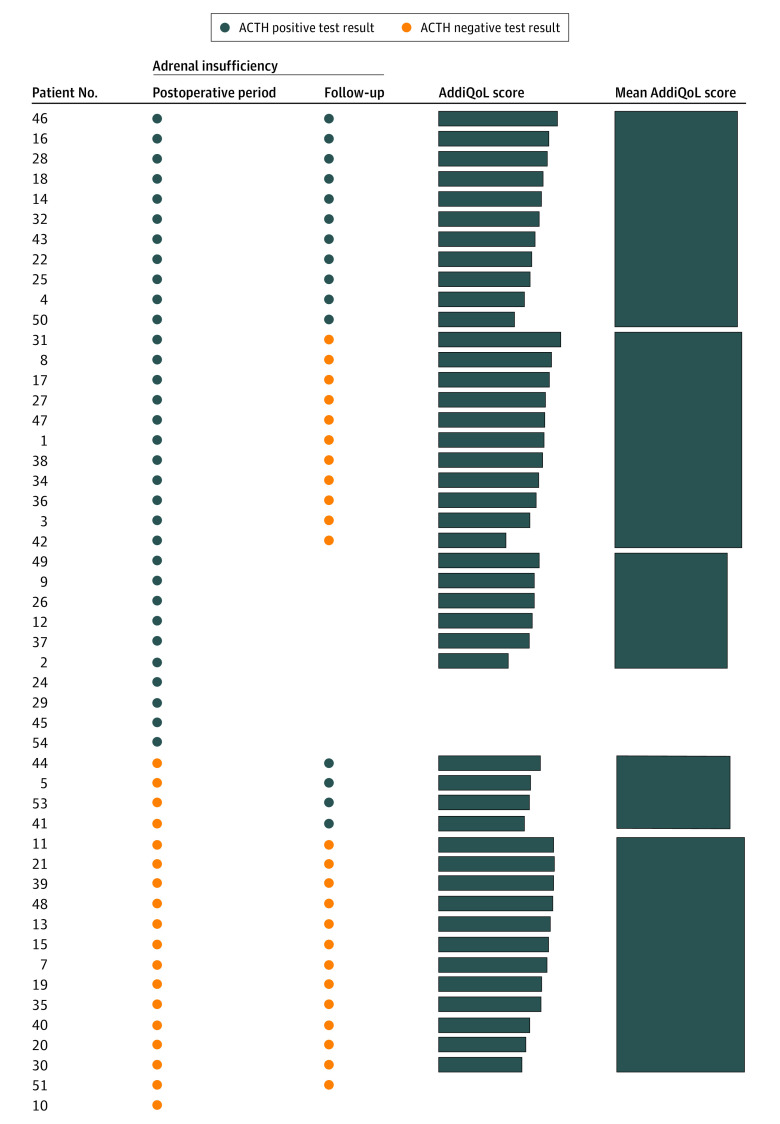
After a median (IQR) follow-up of 13 (9-16) months, 47 patients were alive and disease-free, 2 were alive and undergoing further lines of treatment for recurrent or metastatic disease, and 1 patient died with early tumor recurrence. Among 47 disease-free patients, ACTH stimulation test was repeated at 4 months after surgery and the AddiQoL questionnaire submitted. Four patients could not be reassessed with ACTH test because of intercurrent public health restrictions related to the COVID-19 outbreak, and the response rate for AddiQoL was 97.9% (46 of 47 patients). Late AI was found in 15 of 39 patients (38.5%): in 11 of 15 (73.3%), AI status persisted from the early postoperative period, and in 4 of 15 (26.7%), AI was a new finding. This means that the probability of patients maintaining persistently negative results for AI throughout the study period was 33.3% (13 of 39), the probability of developing an early transient AI was 28.2% (11 of 39), the probability of developing late AI despite normal function at time of hospital discharge was 23.5% (4 of 17), and the probability of developing a persistent AI was 28.2% (11 of 39) (Figure 2).
Figure 2. Results of Adrenocorticotropic Hormone Stimulation Test .
Postoperative includes postoperative days 1 and 10. Health-related Quality of Life in Addison’s Disease (AddiQoL) scores are on a scale from 16.7 to 100.
Even though impaired adrenal function was detected at ACTH test, overall quality of life was not compromised in these patients, as indicated by the fact that lower median (IQR) scores reported in the AddiQoL questionnaire by the group of patients with persistent adrenal insufficiency were not significant (76.1 [72.2-81.1] vs 83.3 [78.1-88.1]; P = .07) (Figure 2). However, patients with AI had a significantly lower median (IQR) score in single items investigating working capacity (question 4 with AI, 4 [4-5], vs without, 5 [4-6]; P = .04), sweating sensation (question 16 with AI, 6 [5-6], vs without, 6 [6-6]; P = .04), and mental concentration (question 24 with AI, 5 [4-6], vs without, 6 [5-6]; P = .03) (eTable 1 in the Supplement).
Secondary Outcomes
Secondary outcomes did not differ between the 2 groups: mean (IQR) operative time (with AI, 436 [374-519] vs without, 459 [431-512]; P = .37), median (IQR) infusion of crystalloids (with AI, 8.51 [7.13-9.67] mL/kg/min vs without, 7.78 [6.57-8.87] mL/kg/h; P = .23), colloids (with AI, 1.24 [0.55-1.55] mL/kg/h vs without, 0.89 [0-1.53] mL/kg/h; P = .25), albumin (with AI, 0.03 [0-0.06] mL/kg/h vs without, 0.04 [0-0.07] mL/kg/h; P = .49), RBC (with AI, 0.76 [0-1.34] mL/kg/h vs without, 0.2 [0-1.13] mL/kg/h; P = .97), and fresh frozen plasma (with AI, 0.2 [0-0] mL/kg/h vs without, 0.14 [0-0] mL/kg/h; P = .66). There was no significant difference between the 2 groups in time-weighted mean (IQR) of MAP less than 65 mm Hg (with AI, 0.74 [0.36-1.24] vs without AI, 1.1 [0.72-1.69]; P = .10) and time-weighted mean (IQR) of SVI less than 35 mL/m2 (with AI, 0.9 [0.18-1.44] vs without AI, 1.08 [0.3-1.51]; P = .92). No difference in the use of vasoactive drugs was observed, in terms of median (IQR) VIS (with AI, 62.0 [46.0-118.8] vs without 122.0 [50.5-159.8]; P = .30) (Table 2). Despite being not significant in the statistical analysis, a trend for higher VIS was found in patients with AI in case of surgical procedures longer than 8 hours: mean (IQR) VIS at 8 hours after incision was 17.7 (6.5-27.5) with AI vs 13.8 (10.0-20.0) without (P = .65); 20.3 (2.5-33.0) vs 16.0 (8.3-24.3) at 9 hours (P = .93); and 27.3 (15.0-41.0) vs 20.5 (15.8-25.8) at 10 hours (P = .80) (Figure 3). There were also no differences in ICU admission (21 patients with AI [65.5%] vs 11 without [61.1]; P = .77), length of ICU stay (mean [IQR] with AI, 1 [1-2] day vs without, 1 [1-2] day; P = .83), and total length of hospital stay (mean [IQR] with AI, 14 [10-18] days vs without, 14 [11-20] days; P = .69). Clavien-Dindo grade 3 or higher complications were not associated with the occurrence of AI (7 patients with AI [21.9%] vs 6 patients without [33.3%]; P = .64) (Table 2).
Figure 3. Mean Vasoactive-Inotropic Score (VIS) Intraoperatively and in the Early Postoperative Period for Patients Admitted to Intensive Care Unit (ICU).
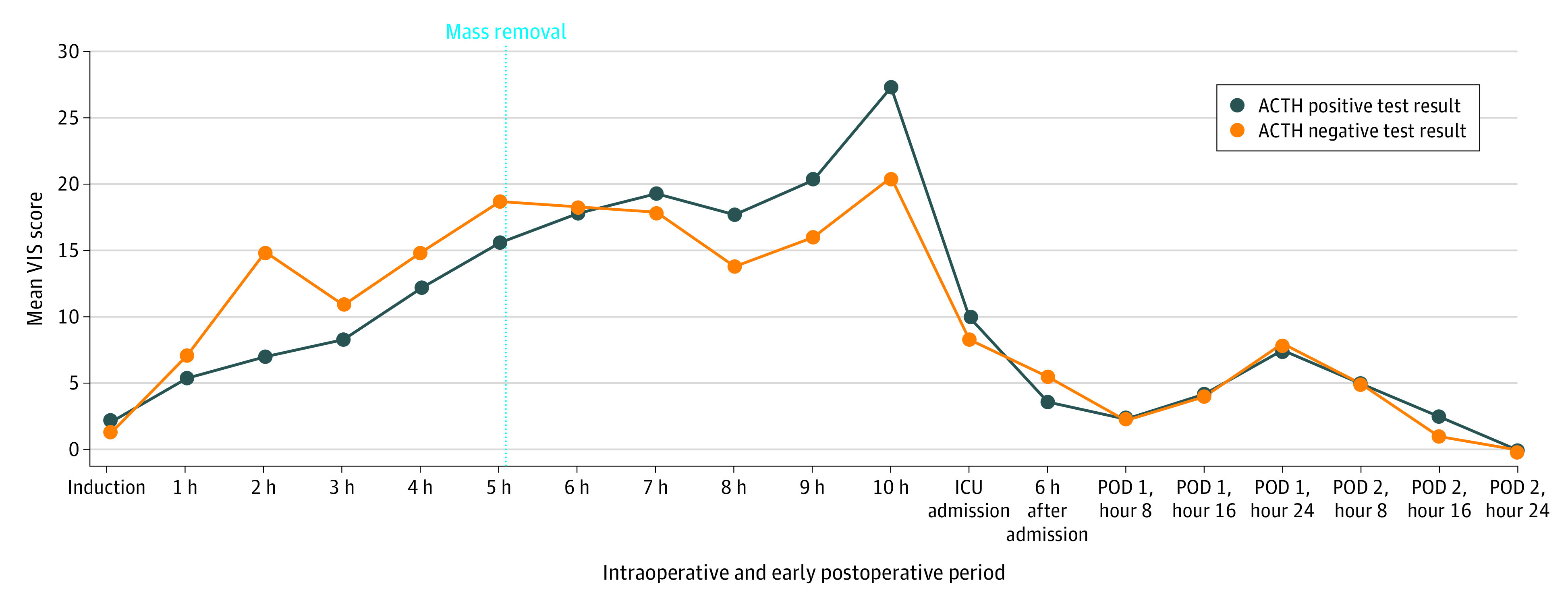
VIS includes any inotropic and vasoactive drug. ACTH indicates adrenocorticotropic hormone; POD, postoperative day.
Discussion
In this prospective study, en bloc adrenalectomy in patients undergoing MVR for primary RPS resulted in adrenal insufficiency in 64% of individuals during the early postoperative period and in 38.5% during long-term follow-up. Patients with AI had significantly higher ASA score and higher malignancy grade tumors. Overall, AI in the early postoperative period did not correlate with ICU admission, need for vasoactive drugs, or postoperative morbidity and mortality. To the best of our knowledge, this is the first study assessing adrenal function in patients undergoing adrenalectomy in the context of major abdominal procedures for a reason other than adrenal disease.
Previously, unexpected AI was reported in literature as occasional event after radical nephrectomy including en bloc resection of the healthy ipsilateral adrenal,15,16,17,18 while in the present study in the context of MVR, AI was found to be a common event. While resection of a healthy adrenal gland should not affect the function and stress response of the hypothalamic-pituitary-adrenocortical axis (HPA) in healthy patients,19 a previously subclinical AI may become evident after major abdominal surgery in 4% to 10% of fragile patients even without removing the adrenal gland.20
Even though the test threshold to diagnose AI is not universally established, the consistency of AI diagnosis in these patients was confirmed by the finding of significantly different basal cortisol values in the postoperative period (Figure 1), as patients diagnosed with AI did not show any increase in cortisol secretion on postoperative day 1. Increase of cortisol values has already been described as part of the stress response in surgical patients, being also proportionally associated with the overall burden of surgery.21 Therefore, the flat curve of basal cortisol values described in patients diagnosed with AI in this study represents further support of the diagnosis.
The finding of a significant correlation between AI and higher ASA score may be related to an insufficient response to surgical stress in frail patients. The association of AI and higher malignancy grade may be related instead to a higher systemic involvement by higher grade tumors, which may reveal an underlying AI.
The counterintuitive finding of patients with AI having fewer organs resected emphasizes that inadequate response to surgical stress is not just explained by the burden of surgical procedure but is more likely associated with patient characteristics and postoperative changes in the HPA axis. While the event of monolateral adrenalectomy did not cause significant effect in most cases, as in nephrectomy or adrenalectomy performed for kidney or adrenal malignancies, the added burden of adrenalectomy in frail patients during a complex MVR may make manifest its importance and its effect on the regulation of postoperative stress response.22 According to our findings, we can postulate that HPA axis–associated stress response is particularly impaired during long surgical procedures. In fact, the increased amount of required vasoactive drugs 8 hours after incision and afterwards in patients with AI may suggest an insufficient stress response (Figure 3).
The finding of significantly higher perivisceral tumor invasion of the removed adrenal in the subgroup of patients without AI is difficult to interpret. Whether the contralateral gland may develop a compensatory mechanism and higher sensibility to ACTH stimulation during tumor growth, leading to more efficient stress response once the gland on the tumor’s side has been removed, may be hypothesized.
The overall relevance of early adrenal insufficiency on postoperative outcome was limited, at least according to measured items. In fact, all considered secondary outcomes failed to identify any major clinical implication in patients developing AI. Specifically, rate of serious complications, ICU admission, length of ICU stay, and total hospital stay were not apparently influenced. Therefore, adrenalectomy was performed without any apparent damage, even in the event of unexpected (and unaware, if not investigated) AI.
While the clinical impact of AI in the early postoperative period was negligible, this condition may endure months after surgery. Only 28% of patients never experienced a period of AI, while 38.5% still showed positive ACTH test results 4 months after surgery. Quality of life evaluated in the long term did not reveal any significant impairment, even though single items investigating working, mental capacity, and sweating sensation showed some reduction. Whether this might suggest corticosteroid replacement therapy in these patients was beyond the aim of present study and merits future investigation.
Limitations
Several limitations of the study need to be acknowledged. First, the small sample size was established a priori to allow an acceptable uncertainty interval, given that an exact a priori estimate of AI incidence was not available. Second, low-dose ACTH stimulation test (1 μg of corticotropin) was adopted as the criterion standard for diagnosis of AI8,23 even in the absence of a universally accepted threshold for test result positivity (peak cortisol value ranging from 18 to 20 μg/dL).9,24 The higher threshold was chosen as to not miss any event. As a results, the risk of false positive results is known to be higher, including the higher chance of diagnosing subclinical conditions. To amend this, a second analysis was run considering a stricter threshold of 18 µg/dL; no changes in significance of postoperative outcomes was found between patients affected by adrenal insufficiency or not. Therefore, regardless of the chosen threshold of the ACTH stimulation test, the clinical consequences of adrenal insufficiency did not show a significant impact in patients undergoing MVR with en bloc adrenalectomy. Patients with known preoperative adrenal impairment, inadequate basal values of cortisol, and ongoing steroid therapy were excluded from the study to avoid confounders. Among screened patients, 4 of 67 (6%) were unaware of their preexisting condition of impaired adrenal function; whether these patients would have experienced clinically relevant acute AI is not known. This question still needs to be answered and is planned to be addressed in future investigations based on the present findings.
Conclusions
Early postoperative AI is common in patients undergoing adrenalectomy in the context of multivisceral resection and may persist until 4 months after surgery. Despite this, findings from this study suggest that adrenalectomy can be safely performed, since morbidity and length of hospital were not affected. We therefore suggest that the extent of resection in retroperitoneal sarcoma should be tailored only based on the extent and biology of disease and not on the hypothetical burden of an en bloc adrenalectomy.
In addition, we suggest that patients candidate to a major surgical procedure, including en bloc adrenalectomy, should be screened for preexisting subclinical impairment of adrenal function and adequately monitored, as the impact of adrenalectomy is not yet known for those who are previously affected by adrenal dysfunction. Adrenal function should be monitored in the early postoperative period and in the long term, especially in frail patients, to identify situations wherein replacement therapy may be beneficial.
eFigure 2. Detailed hemodynamic algorithm for intraoperative and postoperative management
eFigure 1. Study consort diagram
eTable 1. Quality of life in patients with or without long-term AI after multivisceral retroperitoneal resection
eTable 2. Vasoactive Inotropic Score
References
- 1.Bonvalot S, Miceli R, Berselli M, et al. Aggressive surgery in retroperitoneal soft tissue sarcoma carried out at high-volume centers is safe and is associated with improved local control. Ann Surg Oncol. 2010;17(6):1507-1514. doi: 10.1245/s10434-010-1057-5 [DOI] [PubMed] [Google Scholar]
- 2.Swallow CJ, Strauss DC, Bonvalot S, et al. ; Transatlantic Australasian RPS Working Group (TARPSWG) . Management of primary retroperitoneal sarcoma (RPS) in the adult: an updated consensus approach from the Transatlantic Australasian RPS Working Group. Ann Surg Oncol. 2021;28(12):7873-7888. doi: 10.1245/s10434-021-09654-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callegaro D, Fiore M, Gronchi A. Personalizing surgical margins in retroperitoneal sarcomas. Expert Rev Anticancer Ther. 2015;15(5):553-567. doi: 10.1586/14737140.2015.1028375 [DOI] [PubMed] [Google Scholar]
- 4.Dingley B, Fiore M, Gronchi A. Personalizing surgical margins in retroperitoneal sarcomas: an update. Expert Rev Anticancer Ther. 2019;19(7):613-631. doi: 10.1080/14737140.2019.1625774 [DOI] [PubMed] [Google Scholar]
- 5.Gronchi A, Strauss DC, Miceli R, et al. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS): a report on 1007 patients from the multi-institutional collaborative RPS Working Group. Ann Surg. 2016;263(5):1002-1009. doi: 10.1097/SLA.0000000000001447 [DOI] [PubMed] [Google Scholar]
- 6.Callegaro D, Swallow C. ASO author reflections: every step counts: improved survival of retroperitoneal sarcoma patients during the past 15 years. Ann Surg Oncol. 2021;28(3):1710-1711. doi: 10.1245/s10434-020-09119-9 [DOI] [PubMed] [Google Scholar]
- 7.MacNeill AJ, Fiore M. Surgical morbidity in retroperitoneal sarcoma resection. J Surg Oncol. 2018;117(1):56-61. doi: 10.1002/jso.24902 [DOI] [PubMed] [Google Scholar]
- 8.Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364-389. doi: 10.1210/jc.2015-1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mongioì LM, Condorelli RA, Barbagallo F, Cannarella R, La Vignera S, Calogero AE. Accuracy of the low-dose ACTH stimulation test for adrenal insufficiency diagnosis: a re-assessment of the cut-off value. J Clin Med. 2019;8(6):806. doi: 10.3390/jcm8060806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Øksnes M, Bensing S, Hulting AL, et al. Quality of life in European patients with Addison’s disease: validity of the disease-specific questionnaire AddiQoL. J Clin Endocrinol Metab. 2012;97(2):568-576. doi: 10.1210/jc.2011-1901 [DOI] [PubMed] [Google Scholar]
- 11.Salmasi V, Maheshwari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126(1):47-65. doi: 10.1097/ALN.0000000000001432 [DOI] [PubMed] [Google Scholar]
- 12.Belletti A, Lerose C, Zangrillo A, Landoni G. Vasoactive-inotropic score: evolution, clinical utility, and pitfalls. J Cardiothorac Vasc Anesth. 2021;35(10):3067-3077. doi: 10.1053/j.jvca.2020.09.117 [DOI] [PubMed] [Google Scholar]
- 13.MacNeill AJ, Gronchi A, Miceli R, et al. Postoperative morbidity after radical resection of primary retroperitoneal sarcoma: a report from the Transatlantic RPS Working Group. Ann Surg. 2018;267(5):959-964. doi: 10.1097/SLA.0000000000002250 [DOI] [PubMed] [Google Scholar]
- 14.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henrich WL, Goldberg J, Lucas M, Gabow P. Adrenal insufficiency after unilateral radical nephrectomy. Urology. 1976;8(6):584-585. doi: 10.1016/0090-4295(76)90525-2 [DOI] [PubMed] [Google Scholar]
- 16.Safir MH, Smith N, Hansen L, Kozlowski JM. Acute adrenal insufficiency following unilateral radical nephrectomy: a case report. Geriatr Nephrol Urol. 1998;8(2):101-102. doi: 10.1023/A:1008305627588 [DOI] [PubMed] [Google Scholar]
- 17.Karamchandani K, Leathem J, Sinz EH. Acute adrenal insufficiency in the perioperative period: a case report. A A Pract. 2019;12(3):63-65. doi: 10.1213/XAA.0000000000000846 [DOI] [PubMed] [Google Scholar]
- 18.Merry WH, Caplan RH, Wickus GG, et al. Postoperative acute adrenal failure caused by transient corticotropin deficiency. Surgery. 1994;116(6):1095-1100. [PubMed] [Google Scholar]
- 19.Bischoff P, Noldus J, Harksen J, Bause HW. Zur Notwendigkeit der perioperative Kortisolsubstitution. Spontane und stimulierte ACTH—und Kortisolsekretion nach unilateraler Adenalektomie beim Nierenzellkarzinom. The necessity for perioperative cortisol substitution. spontaneous and stimulated ACTH and cortisol secretion during unilateral adrenalectomy for renal cell carcinoma. Anaesthesist. 1997;46(4):303-308. doi: 10.1007/s001010050405 [DOI] [PubMed] [Google Scholar]
- 20.Richards ML, Caplan RH, Wickus GG, Lambert PJ, Kisken WA. The rapid low-dose (1 microgram) cosyntropin test in the immediate postoperative period: results in elderly subjects after major abdominal surgery. Surgery. 1999;125(4):431-440. doi: 10.1016/S0039-6060(99)70011-5 [DOI] [PubMed] [Google Scholar]
- 21.Khoo B, Boshier PR, Freethy A, et al. Redefining the stress cortisol response to surgery. Clin Endocrinol (Oxf). 2017;87(5):451-458. doi: 10.1111/cen.13439 [DOI] [PubMed] [Google Scholar]
- 22.Veenhof AA, Vlug MS, van der Pas MH, et al. Surgical stress response and postoperative immune function after laparoscopy or open surgery with fast track or standard perioperative care: a randomized trial. Ann Surg. 2012;255(2):216-221. doi: 10.1097/SLA.0b013e31824336e2 [DOI] [PubMed] [Google Scholar]
- 23.Ospina NS, Al Nofal A, Bancos I, et al. ACTH stimulation tests for the diagnosis of adrenal insufficiency: systematic review and meta-analysis. J Clin Endocrinol Metab. 2016;101(2):427-434. doi: 10.1210/jc.2015-1700 [DOI] [PubMed] [Google Scholar]
- 24.Karaca Z, Tanriverdi F, Atmaca H, et al. Can basal cortisol measurement be an alternative to the insulin tolerance test in the assessment of the hypothalamic-pituitary-adrenal axis before and after pituitary surgery? Eur J Endocrinol. 2010;163(3):377-382. doi: 10.1530/EJE-10-0229 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 2. Detailed hemodynamic algorithm for intraoperative and postoperative management
eFigure 1. Study consort diagram
eTable 1. Quality of life in patients with or without long-term AI after multivisceral retroperitoneal resection
eTable 2. Vasoactive Inotropic Score