Key Points
Question
Can Holter monitoring be reliably used to dynamically stratify arrhythmic risk in patients with arrhythmogenic right ventricular cardiomyopathy (ARVC) at follow-up?
Findings
In this cohort study including 169 patients with definite ARVC, parameters derived from follow-up Holter monitoring (overall 24-hour premature ventricular contractions [PVC] burden, presence of PVC spikes, and presence of nonsustained ventricular tachycardia) were associated with the overall risk of ventricular arrhythmias during the next 12 months.
Meaning
These findings suggest that a follow-up strategy using yearly Holter monitoring can be used to dynamically assess the arrhythmic risk of patients with ARVC.
This cohort study describes changes in premature ventricular contraction burden over time and assesses whether a changing burden on follow-up Holter monitoring is associated with subsequent arrhythmic events in patients with arrhythmogenic right ventricular cardiomyopathy.
Abstract
Importance
A high burden of premature ventricular contractions (PVCs) at disease diagnosis has been associated with an overall higher risk of ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy (ARVC). Data regarding dynamic modification of PVC burden at follow-up with Holter monitoring and its impact on arrhythmic risk in ARVC are scarce.
Objective
To describe changes in the PVC burden and to assess whether serial Holter monitoring is dynamically associated with sustained ventricular arrhythmias during follow-up in patients with ARVC.
Design, Settings, and Participants
In this cohort study, patients with a definite ARVC diagnosis, available Holter monitoring results at disease diagnosis, and at least 2 additional results of Holter monitoring during follow-up were enrolled from 6 ARVC registries in North America and Europe. Data were collected from June 1 to September 15, 2021.
Main Outcomes and Measures
The association between prespecified variables retrieved at each Holter monitoring follow-up (ie, overall PVC burden; presence of sudden PVC spikes, defined as absolute increase in PVC burden ≥5000 per 24 hours or a relative ≥75% increase, with an absolute increase of ≥1000 PVCs; presence of nonsustained ventricular tachycardia [NSVT]; and use of β-blockers and class III antiarrhythmic drugs) and sustained ventricular arrhythmias occurring within 12 months after that Holter examination was assessed using a mixed logistical model.
Results
In 169 enrolled patients with ARVC (mean [SD] age, 36.3 [15.0] years; 95 men [56.2%]), a total of 723 Holter examinations (median, 4 [IQR, 4-5] per patient) were performed during a median follow-up of 54 (IQR, 42-63) months and detected 75 PVC spikes and 67 sustained ventricular arrhythmias. The PVC burden decreased significantly from the first to the second Holter examination (mean, 2906 [95% CI, 1581-4231] PVCs per 24 hours; P < .001). A model including 24-hour PVC burden (odds ratio [OR] 1.50 [95% CI, 1.10-2.03]; P = .01), PVC spikes (OR, 6.20 [95 CI, 2.74-13.99]; P < .001), and NSVT (OR, 2.29 [95% CI, 1.10-4.51]; P = .03) at each follow-up Holter examination was associated with sustained ventricular arrhythmia occurrence in the following 12 months.
Conclusions and Relevance
These findings suggest that in patients with ARVC, changes in parameters derived from each Holter examination performed during follow-up are associated with the risk of sustained ventricular arrhythmias within 12 months of disease diagnosis.
Introduction
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited cardiac disease characterized by ventricular arrhythmias, an increased risk of sudden cardiac death, and progressive fibrofatty replacement of the myocardium.1,2 The first step in disease management after ARVC diagnosis is risk stratification to determine whether placement of an implantable cardioverter-defibrillator (ICD) for primary prevention is warranted.3,4,5 Recently, a novel risk stratification calculator was developed (https://www.arvcrisk.com).6,7 This calculator allows clinicians to determine the 5-year arrhythmic risk of patients without previous sustained ventricular arrhythmias at the time of diagnosis. Several external cohorts have subsequently confirmed the accuracy of this risk calculator.6,8,9,10,11
Premature ventricular contractions (PVCs) are a hallmark of ARVC. A high PVC burden has also been associated with an increased risk of sustained ventricular arrhythmias and ICD interventions in patients with ARVC.12 Therefore, the assessment of the PVC burden at the time of disease diagnosis is recommended in current clinical risk stratification strategies.3,4,5 In addition, PVC count on a Holter monitor represents one of the components of the recently developed ARVC risk calculator.6,7 However, ARVC is a progressive disease, and the weight of risk markers can vary during follow-up.13 Whether individual variations in PVC burden are associated with future arrhythmic events has not been investigated previously. Thus, the purpose of this study was 2-fold: (1) to describe changes in PVC burden over time in patients with ARVC after disease diagnosis and (2) to determine whether a changing PVC burden on follow-up Holter monitoring is associated with subsequent arrhythmic events.
Methods
Study Design
The ARVC registries of 6 high-volume referral academic institutions (The Johns Hopkins University, Baltimore, Maryland, US; Montreal Heart Institute, Montreal, Quebec, Canada; Ospedale Universitario Careggi, Florence, Italy; Centro Cardiologico Monzino, Milan, Italy; Ospedali Riuniti, Ancona, Italy; and Azienda Sanitaria Universitaria Giuliano Isontina, Trieste, Italy) from 3 different countries were screened for all consecutive patients fulfilling the following inclusion criteria:
Definite ARVC diagnosis in accordance with the 2010 International Task Force Criteria14;
Availability of findings of a 24-hour Holter monitor at disease diagnosis, defined as baseline Holter findings; and
Availability of at least 2 additional Holter findings during the 5 years after disease diagnosis, with a maximum 18-month interval between any 2 Holter findings.
Data were collected from June 1 to September 15, 2021. Ethical review board approval and written patient consent were obtained at each center, in accordance with local regulations. The study was performed in accordance with the Declaration of Helsinki.15 Data supporting these findings are available on reasonable request to the corresponding author. This cohort study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Data Collection
For each patient fulfilling inclusion criteria, demographic characteristics (age, sex, and proband status), clinical history (any ventricular arrhythmia or cardiac syncope preceding disease diagnosis), and ARVC diagnostic features (2010 International Task Force Criteria fulfillment and right ventricular ejection fraction at disease diagnosis retrieved as per previous methods from this group6) were extracted. For patients with available genetic testing results, pathogenic or likely pathogenic variants in one of the genes associated with ARVC were reported after adjudication according to the American College of Medical Genetics and Genomics guidelines.16 From every available Holter finding, the 24-hour PVC burden and the presence of nonsustained ventricular tachycardia (NSVT) and/or sustained ventricular arrhythmia was collected.
The use of classes Ic to III antiarrhythmic drugs (AADs) and β-blockers was assessed at baseline and at every Holter follow-up. Sustained ventricular arrhythmia events occurring during follow-up were recorded. All time-dependent variables were collected with the time of disease diagnosis set as time zero reference. Patient follow-up started at the time of disease diagnosis and ended 12 months after the last available Holter findings, with follow-up completed on January 15, 2021.
Definitions and Study End Points
The primary aims of this study were:
To describe the variation of the PVC burden over time in a population of patients with definite ARVC and
To assess the dynamic association of Holter monitor–derived parameters with the occurrence of a sustained ventricular arrhythmia event in the 12 months immediately after each Holter finding.
We also repeated these analyses with patients stratified according to their history of sustained ventricular arrhythmia at the time of ARVC diagnosis (primary prevention vs secondary prevention) as a secondary aim.
Sustained ventricular arrhythmia was defined as a ventricular tachycardia lasting at least 30 seconds or with hemodynamic compromise requiring cardioversion, ventricular fibrillation and/or flutter, or an appropriate ICD intervention. A PVC spike was defined as (1) an absolute increase in PVC burden of at least 5000 PVCs and/or (2) a relative increase of at least 75% from the preceding Holter monitor finding, with an absolute increase of at least 1000 PVCs. The presence of a PVC spike was assessed for every Holter finding available at follow-up on comparison with the PVC burden of the Holter monitor finding immediately preceding. Sensitivity analyses using 50% and 100% relative burden increase for defining PVC spikes are included in the eMethods in the Supplement.
Statistical Analysis
All analyses were performed using Stata, version 14.0 (StataCorp LLC). Categorical variables were reported as count (percentage). Distribution of continuous variables was tested using a Shapiro-Wilk test. Continuous variables were reported as mean (SD) or as median (IQR), in accordance with variable distribution. Comparisons between numerical variables were performed using a paired t test or Wilcoxon signed rank test. Overall progression of the PVC burden over time was tested through linear regression.
Associations between predetermined clinical and Holter monitor–derived variables of interest (ie, male sex, overall PVC burden, presence of a PVC spike, presence of NSVT, use of β-blockers, and use of class III AADs) and the occurrence of a sustained ventricular arrhythmia event in the subsequent 12 months were tested using mixed-effects logistic regression. Variables of interest were treated as fixed effects, whereas patient identity was treated as a random effect to control for interpatient variability. Only variables reaching significance of 2-sided P < .05 in the single-variable models were considered for inclusion in a subsequent multivariate model. Variables included in the multivariate, mixed-effects logistic regression model were chosen using stepwise, backward selection associated with outcomes and minimization of the Akaike information criterion (with a difference of 2 used as a threshold for continued addition of variables). The discriminative performance of the final model was measured using the C statistic (area under the receiver operating characteristic curve). Agreement between estimated and observed outcomes was evaluated graphically using calibration plots. The predictive capability of the final model was then graphically expressed through predictive curves derived from the fixed margins of the final multivariate mixed-effect logistic model using Stata’s margin function.
Results
Overall Patient Cohort
A total of 169 patients were enrolled in the study. Table 1 summarizes the baseline characteristics of the study cohort. The mean (SD) age was 36.3 (15.0) years; 95 patients (56.2%) were men and 74 (43.8%) were women. Race and ethnicity were not collected for this study. No current data point toward a significant modulating impact of race or ethnicity on outcomes and, as such, this was not considered a variable for this report. At baseline, 86 patients (50.9%) were receiving β-blockers and 39 (23.1%) were receiving class Ic to III AADs. Among the enrolled patients, a total of 723 Holter examinations were performed (median number per patient, 4 [IQR, 4-5]). The median time between Holter examinations was 12 (IQR, 11-15) months, whereas the median follow-up was 54 (IQR, 42-63) months. Table 2 summarizes specific Holter findings of the study cohort.
Table 1. Baseline Patient Characteristics.
| Characteristic | Data (N = 169)a |
|---|---|
| Age, mean (SD), y | 36.3 (15.0) |
| Sex | |
| Men | 95 (56.2) |
| Women | 74 (43.8) |
| Proband status | 128/169 (75.7) |
| 2010 TFC fulfillment | |
| Class I: morphology | |
| Major | 78 (46.2) |
| Minor | 35 (20.7) |
| Class II: tissue characterization | |
| Major | 27 (16.0) |
| Minor | 5 (3.0) |
| Class III: repolarization abnormalities | |
| Major | 89 (52.7) |
| Minor | 50 (29.6) |
| Class IV: depolarization abnormalities | |
| Major | 9 (5.3) |
| Minor | 46 (27.2) |
| Class V: arrhythmias | |
| Major | 31 (18.3) |
| Minor | 101 (59.8) |
| Class VI: family history | |
| Major | 96 (56.8) |
| Minor | 13 (7.7) |
| Pathogenic/likely pathogenic variation | 85 (50.3) |
| PKP2 | 54 (32.0) |
| DSP | 19 (11.2) |
| DSG2 | 8 (4.7) |
| DES | 2 (1.2) |
| FLNC | 2 (1.2) |
| Recent cardiac syncope | 24 (14.2) |
| TWI, median (IQR) | 3 (2-4) |
| NSVT at diagnosis | 61 (36.1) |
| 24-h PVC count, median (IQR) | 5852 (4409-7295) |
| History of sustained ventricular tachycardia at diagnosis | 47 (27.8) |
| RVEF at CMR, mean (SD), % | 46.0 (12.2) |
| Treatment at baseline | |
| β-Blockers | 86 (50.9) |
| AADs | 39 (23.1) |
| Sotalolo | 36 (21.3) |
| Class Ic | 6 (3.5) |
| Amiodarone hydrochloride | 3 (1.8) |
| ICD | 73 (43.2) |
Abbreviations: AADs, antiarrhythmic drugs; CMR, cardiac magnetic resonance; DES, desmin; DSG2, desmoglein 2; DSP, desmoplakin; FLNC, filamin C; ICD, implantable cardioverter-defibrillator; NSVT, nonsustained ventricular tachycardia; PKP2, plakophylin 2; PVC, premature ventricular complex; RVEF, right ventricular ejection fraction; TFC, Task Force Criteria; TWI, T-wave inversion.
Unless otherwise indicated, data are expressed as number (%) of patients.
Table 2. Holter Examination Data.
| Variable | Dataa |
|---|---|
| No. of Holter examinations per patient | |
| Median (IQR) | 4 (4-5) |
| 3 | 42/169 (24.9) |
| 4 | 59/169 (34.9) |
| 5 | 47/169 (27.8) |
| 6 | 21/169 (12.4) |
| Time between Holter examinations, median (IQR), mo | 12 (11-15) |
| Patients experiencing PVC spikes | 69/169 (39.6) |
| Holter monitoring distribution | |
| Baseline | 169/169 (100) |
| 24-h PVC burden, mean (95% CI) | 5852 (4409-7295) |
| NSVT | 61/169 (36.7) |
| Use of β-blockers | 86/169 (50.9) |
| Use of class Ic to III AADs | 39/169 (23.1) |
| Follow-up <12 mo | 146/169 (86.4) |
| 24-h PVC burden, mean (95% CI) | 3248 (2439-4057) |
| PVC spike | 10/146 (6.8) |
| NSVT | 40/146 (27.4) |
| Use of β-blockers | 79/146 (54.1) |
| Use of class Ic to III AADs | 35/146 (24.0) |
| Follow-up 12-24 mo | 149/169 (88.2) |
| 24-h PVC burden, mean (95% CI) | 3477 (2561-4393) |
| PVC spike | 27/149 (18.1) |
| NSVT | 48/149 (32.2) |
| Use of β-blockers | 81/149 (54.4) |
| Use of class Ic to III AADs | 37/149 (24.8) |
| Follow-up 25-36 mo | 122/169 (72.2) |
| 24-h PVC burden, mean (95% CI) | 2838 (2165-3510) |
| PVC spike | 13/122 (10.7) |
| NSVT | 33/122 (27.0) |
| Use of β-blockers | 59/122 (48.4) |
| Use of class Ic to III AADs | 30/122 (24.6) |
| Follow-up 37-48 mo | 86/169 (50.9) |
| 24-h PVC burden, mean (95% CI) | 2824 (1904-3745) |
| PVC spike | 14/86 (16.3) |
| NSVT | 18/86 (20.9) |
| Use of β-blockers | 43/86 (50.0) |
| Use of class Ic to III AADs | 22/86 (25.6) |
| Follow-up 49-60 mo | 51/169 (30.2) |
| 24-h PVC burden, mean (95% CI) | 3564 (2332-4795) |
| PVC spike | 11/51 (21.6) |
| NSVT | 13/51 (25.5) |
| Use of β-blockers | 26/51 (51.0) |
| Use of class Ic to III AADs | 11/51 (21.6) |
Abbreviations: AADs, antiarrhythmic drugs; NSVT, nonsustained ventricular tachycardia; PVC, premature ventricular complex.
Unless otherwise indicated, data are expressed as number/total number (%) of patients.
PVC Burden Variation and PVC Spikes
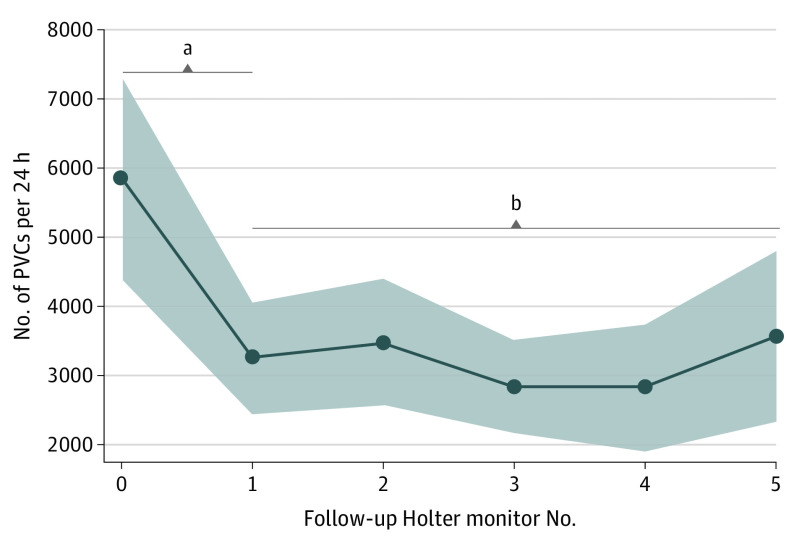
Figure 1 shows the median PVC count per 24 hours on the baseline and subsequent Holter findings obtained during follow-up. The study cohort presented with a high PVC burden at disease diagnosis (mean, 5852 [95% CI, 4409-7295] PVCs per 24 hours). A significant reduction in the 24-hour PVC burden was observed at the first follow-up Holter examination performed at a median of 6 (IQR, 6-12) months from disease diagnosis (mean reduction, 2906 [95% CI, 1581-4231] PVCs per 12 hours; P < .001). After this initial reduction, the 24-hour PVC burden remained stable during subsequent Holter examinations (mean PVC counts at subsequent follow-up: 3477 [95% CI, 2561-4393], 2832 [95% CI, 2165-3510], 2824 [95% CI, 1904-3744], and 3564 [95% CI, 2332-4795]; overall P = .88) (Figure 1). No differences in PVC burden reduction between patients with and without β-blocker therapy were observed (eFigure 1 in the Supplement).
Figure 1. 24-Hour Premature Ventricular Contraction (PVC) Burden Assessed Using Holter Monitoring During Follow-up.
After an initial decrease, the PVC burden remained stable over time. All 169 patients underwent baseline Holter monitoring at disease diagnosis; 146, within the first 12 months after diagnosis; 149, 13 to 24 months after diagnosis; 122, 25 to 36 months after diagnosis; 86, 37 to 48 months after diagnosis; and 50, 49 to 60 months after diagnosis.
aP < .001.
bP = .88.
A total of 75 PVC spikes were identified in 67 (39.6%) of the 169 patients enrolled in this study (32 fulfilling both definitions 1 and 2 for a spike; 43 fulfilling definition 2 for a spike). In Holter findings in which a PVC spike was observed, the median increase in PVC per 24-hour burden was 4900 (IQR, 2400-7139) PVCs per 24 hours (ie, a median increase of 319% [IQR, 142%-1279%]). Baseline characteristics of patients with and without PVC spikes at follow-up were comparable, with the exception of PVC burden at baseline, which was higher in the former (median, 3851 [IQR, 1241-9979] vs 1553 [IQR, 366-7000] PVCs per 24 hours; P = .01) (eTable 1 in the Supplement).
Arrhythmic Events Associated With Holter Results
A total of 67 sustained ventricular arrhythmia events in 57 different patients (33.7%) were observed during follow-up (14 sustained ventricular tachycardia; 50 appropriate ICD interventions; 3 ventricular fibrillation and/or flutter). Most of these events (63 [94.0%]) occurred within 12 months of the previous Holter examination, with only 4 events occurring outside the 12-month window (all between disease diagnosis and first follow-up Holter examination). eTable 2 in the Supplement reports the characteristics of patients with and without ventricular arrhythmia events during follow-up. Twenty-two additional ICDs were implanted during the study follow-up.
eTables 3 to 8 in the Supplement reports the association of each tested parameter individually with the occurrence of a sustained ventricular arrhythmia event in the upcoming 12 months. Occurrence of a sustained ventricular arrhythmia event in the 12 months immediately after each Holter examination was associated with greater 24-hour PVC burden (OR per log increase, 2.19 [95% CI, 1.64-2.93]; P < .001), the presence of PVC spikes (OR, 13.07 [95% CI, 6.04-28.31]; P < .001), or episodes of NSVT (OR, 4.11 [95% CI, 2.33-7.24]; P < .001). The combination of these 3 parameters (PVC count, PVC spike, and NSVT) derived from any Holter finding at follow-up demonstrated a good association with sustained ventricular arrhythmia events in the subsequent 12 months (C statistic, 0.891 [95% CI, 0.853-0.929]). eTable 9 in the Supplement reports the final model, including 24-hour PVC burden (odds ratio [OR] 1.50 [95% CI, 1.10-2.03]; P = .01), PVC spikes (OR, 6.20 [95 CI, 2.74-13.99]; P < .001), and NSVT (OR, 2.29 [95% CI, 1.10-4.51]; P = .03) at each follow-up Holter examination.
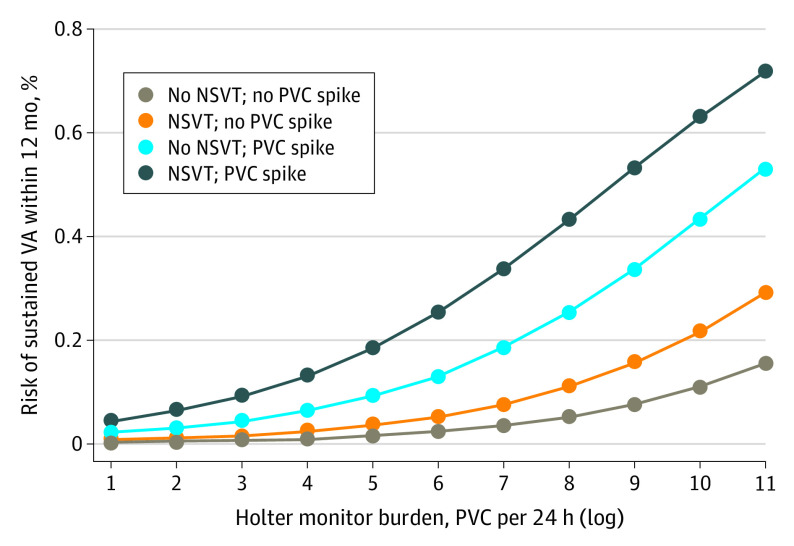
As shown in Figure 2, the risk of a sustained ventricular arrhythmia event within 12 months of a Holter examination increased with the complexity of arrhythmias observed on that set of Holter findings. Notably, however, risk was greater in the presence of PVC spikes (6-fold increase) than NSVT (only 2-fold). For example, a patient with a PVC burden of 3000 PVC per 24 hours (log of approximately 8), NSVT, and a PVC spike would have a greater than 40% risk of a sustained ventricular arrhythmia event within 12 months, whereas a patient with the same PVC burden but without NSVT or the presence of a PVC spike would have an approximately 5% risk. The inclusion of the 24-hour PVC burden at disease diagnosis in the model did not improve its performance. The final model performance in the population stratified by whether the patient had experienced a sustained ventricular arrhythmia at disease diagnosis is reported in eTables 10 and 11 in the Supplement. Model performance in patients with and without ICDs, regardless of their arrhythmic status at baseline, has been reported in eTables 12 and 13 in the Supplement. eFigure 2 in the Supplement reports the calibration plot for the final model.
Figure 2. Association Between Holter Monitor Components and Sustained Ventricular Arrhythmia.
Probability of a sustained ventricular arrhythmia event within 12 months from 24-hour electrocardiographic Holter monitoring performed at follow-up, depending on 3 variables: (1) 24-hour premature ventricular contraction (PVC) burden; (2) presence or absence of nonsustained ventricular tachycardia (NSVT); and (3) presence or absence of a PVC spike. The presence of a PVC spike is associated with a sustained ventricular arrhythmia event in the upcoming 12 months, and these results are clinically more important than the 24-hour PVC burden for any 24-hour Holter findings.
Discussion
To our knowledge, this study is the first to extensively address the changes in the 24-hour PVC burden during follow-up in a multicenter cohort of patients with ARVC and to assess the dynamic association of the parameters derived from follow-up Holter examinations with the occurrence of sustained ventricular arrhythmia events in the subsequent 12 months. The main results of this study may be summarized as follows. First, a significant drop in the overall 24-hour PVC burden within the first 12 months of follow-up from disease diagnosis was observed in most of the patients with ARVC. Second, although the overall PVC burden remained generally stable during the remaining follow-up, the occurrence of sudden, self-limiting increases in PVCs (PVC spikes) at individual Holter examinations was present in approximately one-third of patients. For each Holter examination, both absolute 24-hour PVC burden and presence of PVC spike or NSVT were strongly associated with the occurrence of sustained ventricular arrhythmias in the subsequent 12 months. Together, these results suggest serial Holter monitoring is an effective and accessible strategy that should be considered for dynamic arrhythmia risk assessment and management in patients with ARVC.
Holter Findings Over Time
To our knowledge, this study describes for the first time the modifications in PVC burden observed in patients with ARVC during a long follow-up, including a median of 4 Holter reassessments per patient, performed approximately every 12 months. In our cohort, the PVC burden dropped significantly at the first reassessment after disease diagnosis and remained stable thereafter. This initial PVC burden reduction may be attributed to restriction of endurance and high-level endurance sports and/or initiation of pharmacological therapy. At the time of disease diagnosis and after the first Holter assessment, in fact, 50.9% of patients started β-blocker therapy and 23.1% started class Ic to III AAD therapy, with those percentages remaining stable at all follow-up times. A similar drop in PVC burden has been observed previously in a small cohort of elite athletes with ARVC undergoing physical detraining.10 In that setting, the drop in PVC burden was greater in patients who started β-blocker and/or AAD therapy, but even in patients not receiving medications, a significant reduction was observed. Our findings confirm and extend these findings to a broader population of patients with ARVC. Unfortunately, given the multicentered nature of this study, the dose of physical exercise during follow-up was not routinely quantified in a standardized way. Therefore, the relative weights and the competing benefits of pharmacological treatment and exercise restriction on PVC burden reduction could not be quantified. Further dedicated prospective studies will be required to specifically answer this question.
Sixty-seven patients (39.6%) experienced sudden, self-limiting increases in their PVC burden (PVC spike) on 1 or more follow-up Holter examinations. Arrhythmogenic right ventricular cardiomyopathy is now considered a progressive disease with a relapsing-remitting evolution, with phases of inflammation and increased arrhythmic activity (termed hot phases).17,18,19,20,21 To this day, hot phases have been tracked through the assessment of atypical symptoms at patient admission (ie, myocarditislike episodes), troponin level elevations, cardiac imaging examinations, and histologic assessment,19,21,22,23 of which PVC spikes might represent useful red flags on Holter findings, with a potential impact on management.
Holter PVC Count Association With Arrhythmic Risk
After a definite diagnosis of ARVC, physicians face the complex task of estimating the arrhythmic risk of each individual patient. If this risk is deemed to be low, discontinuation of physical exercise and initiation of β-blocker therapy may be considered an adequate treatment. Conversely, a higher arrhythmic risk may warrant the managing physician discussing the option of implanting an ICD with the patient.
Recently, a novel arrhythmic risk stratification tool for patients with ARVC has been developed.6,7 This tool allows estimation of the 5-year arrhythmic risk of individual patients using clinical and instrumental data, retrieved at the time of disease diagnosis. Still, given the progressive nature of the disease, the profile of patients with ARVC may worsen over time, requiring dynamic risk assessment in primary prevention. In particular, patients deemed at low arrhythmic risk at baseline and thus not receiving an ICD implant may benefit from a dynamic reassessment during follow-up, to facilitate timely capture of sudden changes in arrhythmic propensity and reconsider the indication for a device. Likewise, dynamic risk estimation could help in the management of patients who already have ICD implants, aiding in the titration of β-blocker and AAD therapy to minimize the incidence of appropriate shocks.
The magnitude of PVC burden at disease diagnosis has been associated with the risk of sustained ventricular arrhythmias and ICD interventions in patients with ARVC, and it is part of the recently developed risk calculator.6,12 In this study, we postulated that the arrhythmic data from each Holter monitor examination performed during follow-up, and mainly the observed PVC burden, may be dynamically associated with changes in the arrhythmic risk profile of individual patients. In our cohort, we observed that the overall magnitude of the PVC burden and the presence of PVC spikes and/or NSVT at each follow-up Holter examination were reliably associated with the occurrence of sustained ventricular arrhythmias in the upcoming 12 months, with sudden PVC spikes representing the most important red flag. The presence of PVC spikes was associated with upcoming ventricular arrhythmia events across all subgroups, regardless of ICD status and history of sustained ventricular arrhythmia events at baseline.
The findings of this study support the systematic use of PVC burden reassessment through serial Holter monitoring, to dynamically reevaluate the arrhythmic risk of patients with ARVC during follow-up. Changes in the burden of PVC may be used in clinical practice to integrate the original risk stratification performed at disease diagnosis to assess the need for further changes in lifestyle, pharmacologic management, and an ICD at any point during follow-up. A clinical management strategy integrating a baseline assessment at the time of disease diagnosis using the ARVC risk score calculator and periodic reevaluation can therefore be envisioned.
For patients without a history of sustained ventricular arrhythmias (patients with ARVC undergoing primary prevention), a risk assessment can be performed at disease diagnosis using the ARVC risk calculator. Depending on the predicted risk and through a shared decision-making algorithm accounting for individual values and preferences of the patient, the placement of an ICD in primary prevention may be considered. A yearly reassessment of the arrhythmic risk through Holter monitoring may then be used to evaluate the progression and changes in the arrhythmic profile. This approach may be of particular value when placement of an ICD is not performed at presentation. The results of annual Holter monitoring can then be used to guide further therapeutic interventions such as more aggressive exercise restriction, initiation of or an increase in the dose of β-blocker therapy, initiation of AAD therapy, or reconsideration of the value of ICD implants.
Future Directions and Next Perspectives
Although this dynamic approach seems intuitive and of clinical value, it must be recognized that additional studies are needed to completely assess the impact of Holter monitor–guided management on arrhythmic outcomes. A prospective trial, with the per-protocol yearly performance of Holter monitoring for dynamic arrhythmic risk reassessment and a decision-making algorithm based on Holter findings, represents the next step that needs to be performed. A similar study, including a quantitative exercise exposure assessment and structural (ie, through cardiac ultrasonography or magnetic resonance imaging) and/or serum biomarker (ie, troponin leaks, natriuretic peptide levels) assessments in the presence of a PVC spike would provide additional insights on those points that the present study was not designed to address.
Furthermore, a prospective collection of Holter monitor data in a suitable format for machine-learning analysis and artificial intelligence processing would allow the potential recognition of more exact PVC cutoffs and of even additional variables associated with an increased arrhythmic risk during dynamic follow-up that the naked human eye may have missed. Finally, it should also be noted that several brands of ICDs implement algorithms to quantify the PVC burden of patients with these implants. All PVC burdens presented in our study were derived from Holter findings, and data regarding consistency between Holter monitor–derived and ICD-estimated PVC burdens in the current literature are scarce. Assessing this correlation in future studies will be critical, because it would allow further translation of a dynamic, PVC burden–based arrhythmic risk reassessment into the everyday clinical setting. If a good correlation between Holter monitor–derived and ICD-estimated PVC burden were to be present, it would in fact be reasonable to use continuous ICD estimation of the PVC burden (potentially accessible remotely, during telemedicine visits) to track the changes in the arrhythmic risk of patients with ARVC and an ICD implant, similarly to what is currently done with the estimation of fibrillation burden by implantable loop recorder in the setting of many atrial fibrillation clinics.
Limitations
This study has some limitations, the most prominent of which is its retrospective nature, preventing a fully uniform per-protocol Holter assessment strategy. In addition, although serial monitoring is routinely used at the involved institutions for all patients with ARVC and patients from the entire spectrum of arrhythmic risk in ARVC were included in the study, a certain degree of bias in patient selection cannot be ruled out completely. These findings should therefore be interpreted primarily as hypothesis generating. Finally, because of the retrospective nature of the study, physical exercise modifications during follow-up were not assessed in a standard fashion; therefore, the relative weight of their impact on arrhythmic outcomes and the potential competing benefit with the use of β-blockers and AADs could not be assessed. Further prospective trials building on these findings are therefore needed.
Conclusions
The findings of this multicenter cohort of patients with ARVC suggest that changes in parameters derived from Holter examinations were associated with reduced overall 24-hour PVC burden within 12 months of disease diagnosis. Individual self-limiting PVC spikes were observed in more than one-third of patients, and NSVT was observed on follow-up Holter findings in 20% of patients. The absolute 24-hour PVC burden and the presence of a PVC spike and NSVT at each Holter examination performed during follow-up were associated with the occurrence of ventricular arrhythmias in the 12 months immediately after monitoring. A strategy using yearly Holter monitoring to dynamically assess the individual patient arrhythmic risk profile at follow-up may be an effective integration to the current risk stratification tools for arrhythmic risk in ARVC.
eMethods. Final Model Performance
eTable 1. Characteristics at Disease Diagnosis by Presence of PVC Spikes
eTable 2. Characteristics at Disease Diagnosis by Presence of Ventricular Arrhythmia Events During Follow-up
eTable 3. Association of PVC on Holter Finding With Occurrence of a Sustained Ventricular Arrhythmia Event
eTable 4. Association of PVC Spike on Holter Finding With Occurrence of a Sustained Ventricular Arrhythmia Event
eTable 5. Association of NSVT on Holter Finding With Occurrence of a Sustained Ventricular Arrhythmia Event
eTable 6. Association of Use of β-Blockers During Holter Examination With Occurrence of a Sustained Ventricular Arrhythmia Event
eTable 7. Association of Use of Class III AADs During Holter Examination With Occurrence of a Sustained Ventricular Arrhythmia Event
eTable 8. Association of Male Sex at Holter Examination With Occurrence of a Sustained Ventricular Arrhythmia Event
eTable 9. Results of Final Model
eTable 10. Final Model in Primary Prevention Patients With ARVC (n = 122)
eTable 11. Final Model in Secondary Prevention Patients With ARVC (n = 47)
eTable 12. Final Model Performance in Patients With ARVC and No ICD at Baseline (n = 96)
eTable 13. Final Model Performance in Patients With ARVC Implanted With ICD at Baseline (n = 73)
eFigure 1. PVC Burden Modification During Follow-up Stratifying Patients by β-Blocker Therapy
eFigure 2. Calibration Plots for Final Model
References
- 1.Corrado D, Link MS, Calkins H. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2017;376(1):61-72. [DOI] [PubMed] [Google Scholar]
- 2.Basso C, Corrado D, Marcus FI, Nava A, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373(9671):1289-1300. doi: 10.1016/S0140-6736(09)60256-7 [DOI] [PubMed] [Google Scholar]
- 3.Corrado D, Wichter T, Link MS, et al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Eur Heart J. 2015;36(46):3227-3237. doi: 10.1161/CIRCULATIONAHA.115.017944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calkins H, Corrado D, Marcus F. Risk stratification in arrhythmogenic right ventricular cardiomyopathy. Circulation. 2017;136(21):2068-2082. doi: 10.1161/CIRCULATIONAHA.117.030792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Towbin JA, McKenna WJ, Abrams DJ, et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019;16(11):e301-e372. doi: 10.1016/j.hrthm.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 6.Cadrin-Tourigny J, Bosman LP, Nozza A, et al. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. 2019;40(23):1850-1858. doi: 10.1093/eurheartj/ehz103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cadrin-Tourigny J, Bosman LP, Wang W, et al. Sudden cardiac death prediction in arrhythmogenic right ventricular cardiomyopathy: a multinational collaboration. Circ Arrhythm Electrophysiol. 2021;14(1):e008509. doi: 10.1161/CIRCEP.120.008509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casella M, Gasperetti A, Gaetano F, et al. Long-term follow-up analysis of a highly characterized arrhythmogenic cardiomyopathy cohort with classical and non-classical phenotypes-a real-world assessment of a novel prediction model: does the subtype really matter. Europace. 2020;22(5):797-805. doi: 10.1093/europace/euz352 [DOI] [PubMed] [Google Scholar]
- 9.Aquaro GD, De Luca A, Cappelletto C, et al. Prognostic value of magnetic resonance phenotype in patients with arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2020;75(22):2753-2765. doi: 10.1016/j.jacc.2020.04.023 [DOI] [PubMed] [Google Scholar]
- 10.Gasperetti A, Dello Russo A, Busana M, et al. Novel risk calculator performance in athletes with arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2020;17(8):1251-1259. doi: 10.1016/j.hrthm.2020.03.007 [DOI] [PubMed] [Google Scholar]
- 11.Aquaro GD, De Luca A, Cappelletto C, et al. Comparison of different prediction models for the indication of implanted cardioverter defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy. ESC Heart Fail. 2020;7(6):4080-4088. doi: 10.1002/ehf2.13019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhonsale A, James CA, Tichnell C, et al. Incidence and predictors of implantable cardioverter-defibrillator therapy in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy undergoing implantable cardioverter-defibrillator implantation for primary prevention. J Am Coll Cardiol. 2011;58(14):1485-1496. doi: 10.1016/j.jacc.2011.06.043 [DOI] [PubMed] [Google Scholar]
- 13.Cappelletto C, Stolfo D, De Luca A, et al. Lifelong arrhythmic risk stratification in arrhythmogenic right ventricular cardiomyopathy: distribution of events and impact of periodical reassessment. Europace. 2018;20(FI1):f20-f29. doi: 10.1093/europace/eux093 [DOI] [PubMed] [Google Scholar]
- 14.Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J. 2010;31(7):806-814. doi: 10.1093/eurheartj/ehq025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 16.Richards S, Aziz N, Bale S, et al. ; ACMG Laboratory Quality Assurance Committee . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. doi: 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauce B, Basso C, Rampazzo A, et al. Clinical profile of four families with arrhythmogenic right ventricular cardiomyopathy caused by dominant desmoplakin mutations. Eur Heart J. 2005;26(16):1666-1675. doi: 10.1093/eurheartj/ehi341 [DOI] [PubMed] [Google Scholar]
- 18.Friedrich MG, Sechtem U, Schulz-Menger J, et al. ; International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis . Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol. 2009;53(17):1475-1487. doi: 10.1016/j.jacc.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martins D, Ovaert C, Khraiche D, Boddaert N, Bonnet D, Raimondi F. Myocardial inflammation detected by cardiac MRI in arrhythmogenic right ventricular cardiomyopathy: a paediatric case series. Int J Cardiol. 2018;271:81-86. doi: 10.1016/j.ijcard.2018.05.116 [DOI] [PubMed] [Google Scholar]
- 20.Basso C, Corrado D, Bauce B, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2012;5(6):1233-1246. doi: 10.1161/CIRCEP.111.962035 [DOI] [PubMed] [Google Scholar]
- 21.Bariani R, Cipriani A, Rizzo S, et al. “Hot phase” clinical presentation in arrhythmogenic cardiomyopathy. Europace. 2021;23(6):907-917. doi: 10.1093/europace/euaa343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casella M, Dello Russo A, Bergonti M, et al. Diagnostic yield of electroanatomic voltage mapping in guiding endomyocardial biopsies. Circulation. 2020;142(13):1249-1260. doi: 10.1161/CIRCULATIONAHA.120.046900 [DOI] [PubMed] [Google Scholar]
- 23.Scheel PJ III, Murray B, Tichnell C, et al. Arrhythmogenic right ventricular cardiomyopathy presenting as clinical myocarditis in women. Am J Cardiol. 2021;145:128-134. doi: 10.1016/j.amjcard.2020.12.090 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Final Model Performance
eTable 1. Characteristics at Disease Diagnosis by Presence of PVC Spikes
eTable 2. Characteristics at Disease Diagnosis by Presence of Ventricular Arrhythmia Events During Follow-up
eTable 3. Association of PVC on Holter Finding With Occurrence of a Sustained Ventricular Arrhythmia Event
eTable 4. Association of PVC Spike on Holter Finding With Occurrence of a Sustained Ventricular Arrhythmia Event
eTable 5. Association of NSVT on Holter Finding With Occurrence of a Sustained Ventricular Arrhythmia Event
eTable 6. Association of Use of β-Blockers During Holter Examination With Occurrence of a Sustained Ventricular Arrhythmia Event
eTable 7. Association of Use of Class III AADs During Holter Examination With Occurrence of a Sustained Ventricular Arrhythmia Event
eTable 8. Association of Male Sex at Holter Examination With Occurrence of a Sustained Ventricular Arrhythmia Event
eTable 9. Results of Final Model
eTable 10. Final Model in Primary Prevention Patients With ARVC (n = 122)
eTable 11. Final Model in Secondary Prevention Patients With ARVC (n = 47)
eTable 12. Final Model Performance in Patients With ARVC and No ICD at Baseline (n = 96)
eTable 13. Final Model Performance in Patients With ARVC Implanted With ICD at Baseline (n = 73)
eFigure 1. PVC Burden Modification During Follow-up Stratifying Patients by β-Blocker Therapy
eFigure 2. Calibration Plots for Final Model