Abstract
Background
Discharge planning is a routine feature of health systems in many countries that aims to reduce delayed discharge from hospital, and improve the co‐ordination of services following discharge from hospital and reduce the risk of hospital readmission. This is the fifth update of the original review.
Objectives
To assess the effectiveness of planning the discharge of individual patients moving from hospital.
Search methods
We searched CENTRAL, MEDLINE, Embase and two trials registers on 20 April 2021. We searched two other databases up to 31 March 2020. We also conducted reference checking, citation searching and contact with study authors to identify additional studies.
Selection criteria
Randomised trials that compared an individualised discharge plan with routine discharge that was not tailored to individual participants. Participants were hospital inpatients.
Data collection and analysis
Two review authors independently undertook data analysis and quality assessment using a pre‐designed data extraction sheet. We grouped studies by older people with a medical condition, people recovering from surgery, and studies that recruited participants with a mix of conditions. We calculated risk ratios (RRs) for dichotomous outcomes and mean differences (MDs) for continuous data using fixed‐effect meta‐analysis. When combining outcome data it was not possible because of differences in the reporting of outcomes, we summarised the reported results for each trial in the text.
Main results
We included 33 trials (12,242 participants), four new trials included in this update. The majority of trials (N = 30) recruited participants with a medical diagnosis, average age range 60 to 84 years; four of these trials also recruited participants who were in hospital for a surgical procedure. Participants allocated to discharge planning and who were in hospital for a medical condition had a small reduction in the initial hospital length of stay (MD − 0.73, 95% confidence interval (CI) − 1.33 to − 0.12; 11 trials, 2113 participants; moderate‐certainty evidence), and a relative reduction in readmission to hospital over an average of three months follow‐up (RR 0.89, 95% CI 0.81 to 0.97; 17 trials, 5126 participants; moderate‐certainty evidence). There was little or no difference in participant's health status (mortality at three‐ to nine‐month follow‐up: RR 1.05, 95% CI 0.85 to 1.29; 8 trials, 2721 participants; moderate certainty) functional status and psychological health measured by a range of measures, 12 studies, 2927 participants; low certainty evidence). There was some evidence that satisfaction might be increased for patients (7 trials), caregivers (1 trial) or healthcare professionals (2 trials) (very low certainty evidence). The cost of a structured discharge plan compared with routine discharge is uncertain (7 trials recruiting 7873 participants with a medical condition; very low certainty evidence).
Authors' conclusions
A structured discharge plan that is tailored to the individual patient probably brings about a small reduction in the initial hospital length of stay and readmissions to hospital for older people with a medical condition, may slightly increase patient satisfaction with healthcare received. The impact on patient health status and healthcare resource use or cost to the health service is uncertain.
Keywords: Aged; Aged, 80 and over; Humans; Middle Aged; Hospitals; Length of Stay; Patient Discharge; Patient Readmission; Patient Satisfaction; Randomized Controlled Trials as Topic
Plain language summary
Discharge planning from hospital
What is the aim of this review
The aim of this review was to find out if discharge planning that is tailored to an individual improves the quality of health care delivered by reducing delayed discharge from hospital, reducing transfer back to hospital and improving patients' health status. We also wanted to know how much the intervention cost. We collected and analysed all relevant studies to answer this question. This is the fifth update of the original review.
Key messages
When people leave hospital with a personalised discharge plan there is probably a small reduction in length of stay, they are probably slightly less likely to be admitted to hospital after their discharge from hospital. There is little evidence on the impact on patient health status, patient satisfaction with the care received. The cost of discharge planning is uncertain.
What was studied in the review
Discharge planning is the development of a personalised plan that assesses a patient's health and social care needs prior to them leaving hospital, to support the timely transition between hospital and home or another setting and improve the organisation of post‐discharge services.
What are the main results of the review?
We found 33 trials that compared personalised discharge plans versus standard discharge care. This review indicates that a personalised discharge plan probably leads to a very small reduction in hospital length of stay and probably slightly reduces readmission rates for people who were admitted to hospital with a medical condition, and may increase patient satisfaction. There is little evidence on health status, or the cost of discharge planning to the health service.
How up‐to‐date is this review?
The review authors searched for studies that had been published up to April 2021.
Summary of findings
Summary of findings 1. Effect of discharge planning on patients admitted to hospital.
| Effect of discharge planning on patients admitted to hospital | ||||||
|
Patient or population: patients admitted to hospital with a medical condition (27 trials), with a mix of medical and surgical conditions (4 trials), following a fall (1 trial), with a psychiatric diagnosis (2 trials), with a mix of mental health and medical diagnosis.
Settings: hospital; North America (16 trials), Europe (13 trials), Asia (4 trials), South America (1 trial), Oceania (1 trial)
Intervention: discharge planning Comparison: usual care, mostly with some discharge planning but without a formal link through a coordinator to other departments and services | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Without discharge planning | With discharge planning | |||||
| Hospital length of stay Follow‐up: 3 to 6 months | Study population admitted with a medical condition | (MD ‐0.73, 95% CI ‐1.33 to ‐0.12) |
2113 (11 trials) |
⊕⊕⊕⊝ moderateb |
Gillespie 2009; Harrison 2002; Laramee 2003; Lindpaintner 2013; Moher 1992; Naughton 1994; Naylor 1994; Preen 2005; Rich 1993; Rich 1995; Sulch 2000 | |
| The mean hospital length of stay ranged across control groups from 5.2 to 12.4 daysa | The mean hospital length of stay in the intervention groups was 0.73 lower (95% CI 1.33 to ‐0.12 lower) | |||||
|
Unscheduled readmission Follow‐up: 2 weeks to 6 months |
Study population admitted with a medical condition | RR 0.89 (0.81 to 0.97) | 5126 (17 trials) | ⊕⊕⊕⊝ moderateb | Balaban 2008; Bonetti 2018; Farris 2014; Goldman 2014; Harrison 2002; Jack 2009; Kennedy 1987; Lainscak 2013; Laramee 2003; Legrain 2011; Lisby 2019; Moher 1992; Naylor 1994; Nazareth 2001; Nguyen 2018; Rich 1993; Rich 1995 | |
| 271 per 1,000 | 242 per 1000 (200 to 263) | |||||
| Patient health status | Mortality (follow‐up 3 to 9 months) | |||||
| 110 per 1,000 | 115 per 1,000 | RR 1.05 (0.85 to 1.29) | 2721 (8 studies) | ⊕⊕⊕⊝b moderate |
Goldman 2014; Lainscak 2013; Laramee 2003; Legrain 2011; Nazareth 2001; Nguyen 2018; Rich 1995; Sulch 2000 | |
| Functional status and psychological health (follow‐up 1 to 6 months) | ||||||
| Most studies reported little or no differences between groups for general and disease‐specific health‐related quality of life (Harrison 2002; Kennedy 1987; Lainscak 2013; Lisby 2019; Naylor 1994; Nazareth 2001; Nguyen 2018; Preen 2005; Weinberger 1996; measured with EQ‐5D‐3L, LTCIS, SF‐12, SF‐36, VAS). Two studies that recruited participants with heart failure reported less disability (MLHFQ; MD 8.59, 95% CI 4.02 to 13.16; Cajanding 2017) and better quality of life (CHFQ; MD 22.1, SD 20.8; Rich 1995) for those allocated to the intervention. Sulch 2000 recruited participants recovering from a stroke and reported that those allocated to the intervention scored worse on activities of daily living and quality of life (EQ‐5D), with little or no difference between groups for stroke‐related disability (Rankin score) and anxiety and depression symptoms (HADS). |
2927 (12 studies) | ⊕⊕⊝⊝ lowc |
||||
|
Satisfaction of patients, care givers and healthcare professionals Follow‐up: 2 weeks to 6 months Measured with PSQ, SF‐PSQ‐18, in‐house developed questions |
Four studies reported an increased level of satisfaction for participants allocated to the intervention group (Cajanding 2017; Laramee 2003; Moher 1992; Weinberger 1996), and three little or no difference (Nazareth 2001; (Lindpaintner 2013; Lisby 2019). One small study reported that care givers of participants allocated to the intervention group were more satisfied with the discharge process, and little or no difference for healthcare professionals (Lindpaintner 2013). | 756 participants when reported (8 trials) | ⊕⊝⊝⊝ very lowd |
Satisfaction was measured in different ways (SF‐PSQ‐18 Short‐Form Patient Questionnaire, PSQ Patient Satisfaction Questionnaire) and findings were not consistent across studies; 8/35 studies reported data for this outcome. | ||
| Healthcare resource use and costs | Eleven trials reported findings on an aspect of cost to the health service, it is uncertain whether there is a difference in hospital, primary or community care costs when discharge planning is implemented for patients with a medical condition (Farris 2014; Gillespie 2009; Goldman 2014; Jack 2009; Laramee 2003; Lisby 2019; Naughton 1994; Nazareth 2001; Rich 1995; Weinberger 1996), or who are in hospital for surgery (Naylor 1994). |
5220 participants (11 trials) | ⊕⊝⊝⊝ very lowd |
Healthcare resources that were costed and charges varied among trials. | ||
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CHFQ: Chronic Heart Failure Questionnaire; CI: Confidence interval; EQ‐5D: European Quality of Life Questionnaire; HADS: Hospital Anxiety and Depression scale; LTCIS: Long Term Care Information System; MD: Mean difference; MLHFQ: Minnesota Living With Heart Failure Questionnaire; RR: Risk ratio; SF: Short Form Survey; VAS: Visual Analogue Scale. | ||||||
| GRADE Working Group grades of evidence High:This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different (i.e., large enough to affect a decision) is low. Moderate: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different is moderate. Low: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different is high. Very low: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different is very high. | ||||||
a The range excludes length of stay of 45 days reported by Sulch, due to recruiting participants who were recovering from a stroke and had a longer length of stay.
b We downgraded the evidence to moderate due to imprecision
c We downgraded the evidence to low due to concerns about inconsistency and imprecision
d We downgraded the evidence to very low due to very serious inconsistency and imprecision
Background
A delayed discharge from hospital to home or another setting can lead to poorer patient outcomes, be a cause of distress to patients and their families (Mäkelä 2020), and increase the cost to the health system (Landeiro 2019). Recent trends to support timely discharge from hospital include targeting those patients who incur greater healthcare expenditures, strengthening arrangements for the transition from hospital to home and implementing policies such as discharge planning. Even a small reduction in hospital length of stay and readmission rates could have a substantial financial impact (Burgess 2014; Finkelstein 2020; Sezgin 2020),
Description of the condition
Delayed discharge from hospital occurs when a person is medically fit to be discharged home or another setting, but arrangements for transfer and subsequent care are not in place and the person remains in hospital. Delays can be due to incomplete assessment during the hospital admission, disruption of long‐standing care arrangements, difficulty accessing follow‐up health and social care or poor communication between the hospitals and community health and social care providers (NHS 2020; Bibbins‐Domingo 2019).
Description of the intervention
Discharge planning is the development of an individualised discharge plan for a patient prior to them leaving hospital for home. The discharge plan can be a stand‐alone intervention, may include post‐discharge support (Parker 2002; Phillips 2004) or may be embedded within another intervention. For example, as a component of stroke unit care (Langhorne 2020), as part of comprehensive geriatric assessment (Ellis 2017) or it may be part of a medicine review at the time a person transitions from hospital to home (Redmond 2016). Over the years there has been increased attention on medication errors that can occur at the time of discharge from hospital, with evidence indicating that errors are more likely to occur when a patient is transferred from one healthcare setting to another during admission (WHO 2019).
How the intervention might work
The aim of discharge planning is to improve the efficiency and quality of healthcare delivery by reducing delayed discharge from hospital, facilitating the transition of patients from hospital to a post‐discharge setting and providing patients with information about the management of their health problems. There is evidence to suggest that discharge planning (i.e. an individualised plan for a patient prior to them leaving hospital for home) combined with additional post‐discharge support can reduce unplanned readmission to hospital for patients with congestive heart failure (Phillips 2004). Discharge planning with or without post‐discharge follow‐up may improve patient outcomes and contain costs, by avoiding a prolonged admission to hospital and strengthening arrangements for subsequent health and social care (Balaban 2008; NHS Long Term Plan 2019). It is possible that discharge planning might have a differential effect for different populations, such as older people with complex healthcare needs compared with people admitted to a mental health facility or recovering from elective surgery. How healthcare is organised might also impact on the effectiveness of discharge planning, procedures may vary between specialities and healthcare professionals across hospitals and within the same hospital (Ubbink 2014).
Why it is important to do this review
Clinical guidance issued by professional and government bodies in the UK (RCP 2017; Dept of Health 2020), the USA (DHHS 2019), Australia (Health Direct 2020) and Canada (Health Qual Ontario 2013) highlight the importance of planning discharge as soon as a person is admitted to hospital, of involving a multidisciplinary team to provide a comprehensive assessment, communication with the patient and their caregivers, shared decision‐making, and liaising with health and social services in the community. We have conducted a systematic review of discharge planning to categorise the different types of study populations and discharge plans being implemented, and to assess the effectiveness of organising services in this way. The focus of this review is the effectiveness of discharge planning implemented in an acute hospital setting. This is the fifth update of the original review.
Objectives
To assess the effectiveness and cost to the health service of planning the discharge of individual patients moving from hospital.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials.
Types of participants
All patients in hospital (acute, rehabilitation or community) irrespective of age, gender or condition.
Types of interventions
We defined discharge planning as the development of an individualised discharge plan for a patient prior to them leaving hospital for home or residential care. Where possible, we divided the process of discharge planning according to the steps identified by Marks 1994:
preadmission assessment (where possible);
case finding on admission;
inpatient assessment and preparation of a discharge plan based on individual patient needs, for example a multidisciplinary assessment involving the patient and their family, and communication between relevant professionals within the hospital;
implementation of the discharge plan, which should be consistent with the assessment and requires documentation of the discharge process;
monitoring in the form of an audit to assess if the discharge plan was implemented.
We excluded studies from the review if they did not include an assessment or implementation phase in the discharge plan; if discharge planning appeared to be a minor part of a multifaceted intervention; or if the focus was on the provision of care after discharge from hospital.
The control group had to receive standard care with no individualised discharge plan.
Types of outcome measures
Primary outcomes
1. Hospital length of stay
2. Unscheduled readmission to hospital
3. Patient health status: mortality, functional status, psychological health
4. Satisfaction of patients, caregivers and healthcare staff
5. Healthcare resource use and costs
Secondary outcomes
6. Medication use for studies evaluating a pharmacist led discharge plan
7. Place of discharge
Search methods for identification of studies
Electronic searches
We searched the following databases on 20 April 2021:
Cochrane Central Register of Controlled Trials (CENTRAL) (2021, Issue 3)
MEDLINE, Ovid (2015 to 20 April 2021)
Embase, Ovid (2015 to 20 April 2021)
CINAHL, EBSCO (2015 to 31 March 2020)
PsycINFO, Ovid (2015 to 31 March 2020)
Searches were revised for this update by evaluating titles, abstracts and index terms (MeSH) of 29 included studies from previous versions of the review using the Yale MeSH analyzer (mesh.med.yale.edu/). Sources which had not yielded any unique studies over a number of iterations of the search were searched for this update in March 2020 but were not searched for the rerun in April 2021 (PsycINFO and CINAHL). Search strategies are comprised of natural language and controlled vocabulary terms. We applied no limits on language. Searches were run from 2015 onwards ‐ the date of publication of the previous version of the review. In databases where it was possible and appropriate, study design filters for randomised trials were used; in MEDLINE we used a modified version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximi zing version (2008 revision) (Lefebvre 2021). Limits were used in Embase to remove MEDLINE records in order to avoid duplication in downloaded results. Remaining results were de‐duplicated in EndNote against each other and against results from searches conducted for previous versions of the review. All search strategies used in this version of the review are provided in Appendix 1. Search strategies and search methods used in previous versions of the review are published within those prior publications.
Searching other resources
We searched two trials registers on 20 April 2021:
US National Institutes of Health trial register (ClinicalTrials.gov)
WHO ICTRP (World Health Organization International Clinical Trials Registry Platform) (trialsearch.who.int/)
We reviewed systematic reviews retrieved by the searches, as well as the reference lists of all included studies. When necessary, we contacted individual trialists to clarify issues and to identify unpublished data.
Data collection and analysis
For this update, we followed the same methods defined in the protocol and used in previous versions of this systematic review. We created a summary of findings table using the following outcomes: unscheduled hospital readmission, hospital length of stay, health status, satisfaction and costs. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and risk of bias) to assess the certainty of the evidence as it relates to the main outcomes (Guyatt 2008). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook (Higgins 2011). We justified all decisions to down‐ or up‐grade the certainty of evidence using footnotes to aid readers' understanding of the review where necessary.
Selection of studies
For this update, two review authors (of DCGB, IC, NL, LC and SS) read the abstracts in the records retrieved by the electronic searches to identify publications that appeared to be eligible for this update, and two (of DCGB, IC, NL, LC, SS) independently assessed the full text of all potentially relevant papers to select studies for inclusion. We settled any disagreements by discussion. For previous versions of this review, please see details of those involved in selecting studies in the Acknowledgements section of this review.
Data extraction and management
For this update, two review authors working independently (DCGB, ACB) extracted data from the studies included in this update using a data extraction form developed by EPOC, modified and amended for the purposes of this review (EPOC 2015); these were reviewed by SS. We extracted information on study characteristics (citation, aim, setting, design, risk of bias, study duration, ethical approval, funding sources), participant characteristics (method of recruitment, inclusion/exclusion criteria, study population health problems and diagnosis, total number, withdrawals and number lost to follow‐up, socio‐demographic indicators), intervention (setting, preadmission assessment, case finding on admission, inpatient assessment and preparation of discharge plan, implementation of discharge plan, monitoring phase, and comparison), and outcomes.
Assessment of risk of bias in included studies
For this update, three review authors (DCGB, ACB or SS) independently assessed risk of bias for random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and baseline data using Cochrane's risk of bias tool (Higgins 2011). Each domain was assessed as being at high, low or unclear risk of bias. Disagreements were resolved by discussion with SS. We prioritised the main outcomes length of stay and readmission for our overall assessment of bias for each study.
Measures of treatment effect
We calculated risk ratios (RRs) for unscheduled readmissions and mortality with 95% confidence intervals (CIs) for all point estimates, values less than 1 indicated outcomes favouring discharge planning. We calculated mean differences (MDs) with 95% CIs for the hospital length of stay, and reported the results from the individual studies for the remaining outcomes.
Unit of analysis issues
All the included studies were parallel randomised trials, where participants were individually allocated to the treatment or control groups.
Dealing with missing data
We contacted investigators for missing data; we did not include unpublished data in this update.
Assessment of heterogeneity
We quantified heterogeneity among trials using the I2 statistic and Cochrane's Q test (Cochran 1954). The I2 statistic quantifies the percentage of the total variation across studies that is due to heterogeneity rather than chance (Higgins 2003); smaller percentages suggest less observed heterogeneity (Higgins 2019).
Assessment of reporting biases
We constructed funnel plots for the meta‐analysis of the main outcomes, hospital length of stay and readmission (Higgins 2019).
Data synthesis
We calculated a summary statistic for each outcome when there were sufficient data, using Review Manager 5.4 (Review Manager 2020). We used a fixed‐effect model unless heterogeneity was detected, using an I2 of greater than 60% as a rough guide of substantial heterogeneity. We used the Sythesis Without Meta‐analysis and EPOC guidance to summarise the findings if it was not possible to combine data for meta‐analysis (Campbell 2020; EPOC 2017), by reporting the range of estimates of effect and level of uncertainty for each outcome.
Subgroup analysis and investigation of heterogeneity
In order to reduce differences between studies, we grouped trial results by participants' condition (medical, requiring surgery, admitted to a mental health facility or studies that recruited participants with a mix of conditions), as the discharge planning needs for these groups might differ. We extracted data on the elements of the intervention with a focus on the timing of the discharge plan, who was the discharge lead, the inclusion of patient education and how the discharge plan was implemented.
Sensitivity analysis
We did not conduct sensitivity analysis.
Summary of findings and assessment of the certainty of the evidence
We created a summary of findings table using GRADEpro GRADEpro GDT 2021) for the main outcomes of hospital length of stay, unscheduled readmission to hospital, patient health status, satisfaction of patients, caregivers and healthcare professionals, healthcare resource use and costs.
Results
Description of studies
Results of the search
We retrieved 4632 results from electronic searches (Figure 1). Of these, we screened the full text of 277 records and describe reasons for excluding 59 of the studies. We excluded one study that had previously been included due the focus on an occupational therapy post‐discharge home visit (Pardessus 2002), we included four new studies in this update (Bonetti 2018; Cajanding 2017; Lisby 2019; Nguyen 2018), and added these to the 29 trials previously identified (Balaban 2008; Bolas 2004; Eggink 2010; Evans 1993; Farris 2014; Gillespie 2009; Goldman 2014; Harrison 2002; Hendriksen 1990; Jack 2009; Kennedy 1987; Kripalani 2012; Lainscak 2013; Laramee 2003; Legrain 2011; Lin 2009; Lindpaintner 2013; Moher 1992; Naji 1999; Naughton 1994; Naylor 1994; Nazareth 2001; Parfrey 1994; Preen 2005; Rich 1993; Rich 1995; Shaw 2000; Sulch 2000; Weinberger 1996), for a total of 33 studies (12,242 participants, average sample size 370 participants). One of the trials included in the review was translated from Danish to English (Hendriksen 1990). Follow‐up times varied from five days to 12 months.
1.
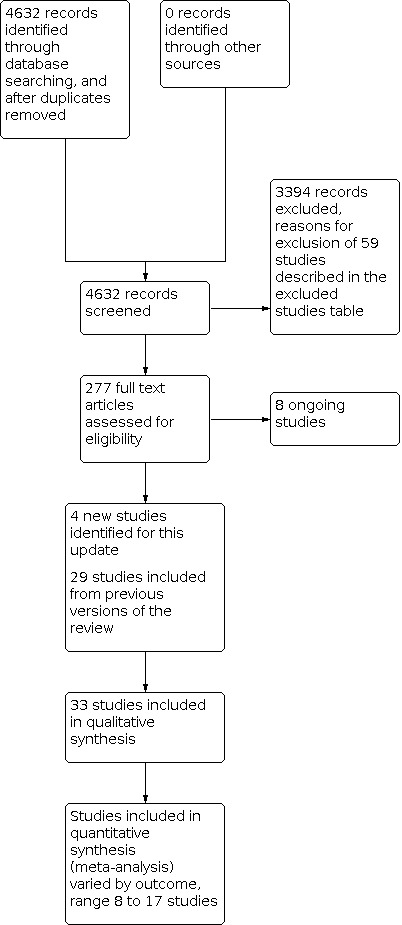
PRISMA flow diagram
Included studies
Twenty‐six of the 33 trials recruited participants with a medical condition (Balaban 2008; Bolas 2004; Bonetti 2018; Cajanding 2017; Eggink 2010; Farris 2014; Gillespie 2009; Goldman 2014; Harrison 2002; Jack 2009; Kennedy 1987; Kripalani 2012; Lainscak 2013; Laramee 2003; Legrain 2011; Lindpaintner 2013; Lisby 2019; Moher 1992; Naughton 1994; Nazareth 2001; Nguyen 2018; Preen 2005; Rich 1993; Rich 1995; Sulch 2000; Weinberger 1996), with an average age range of 60 to 84 years; nine of these trials recruited participants with heart‐related problems (heart failure or acute coronary syndrome) (Bonetti 2018; Cajanding 2017; Eggink 2010; Harrison 2002; Kripalani 2012; Laramee 2003; Nguyen 2018; Rich 1993; Rich 1995), one recruited participants recovering from a stroke (Sulch 2000), and one trial included participants with chronic obstructive pulmonary disease (Lainscak 2013). Four trials recruited participants with a mix of medical and surgical conditions (Evans 1993; Hendriksen 1990; Naylor 1994; Parfrey 1994), one with older people (average age 78 years) admitted to hospital following a hip fracture (Lin 2009), and two with participants who were receiving care in a mental health facility (Naji 1999; Shaw 2000). Two trials used a questionnaire designed to identify participants likely to require discharge planning (Evans 1993; Parfrey 1994). Three trials recruited an ethnically diverse low‐income and under‐served population (Balaban 2008; Goldman 2014; Jack 2009).
The majority of trials evaluated a discharge planning intervention that aimed to facilitate the co‐ordination of post‐discharge care and improve communication between the hospital, primary care and community services to aid the transition of patients from hospital to their discharge destination (see Characteristics of included studies and Table 2). In all but three trials (Evans 1993; Naji 1999; Parfrey 1994), the discharge planning intervention included an education component that provided patients with information of their health condition, medicines and post‐discharge arrangements. In 21 trials a review of medicines was described as one element of the discharge planning intervention, and in nine studies medicine review and reconciliation was the focus of the intervention (Bolas 2004; Bonetti 2018; Eggink 2010; Farris 2014; Gillespie 2009; Kripalani 2012; Nazareth 2001; Nguyen 2018; Shaw 2000).
1. Intervention characteristics.
| Study ID | Components of the assessment and implementation of the discharge plan | Aim, focus and content of the discharge plan | Follow‐up as part of the discharge planning intervention | Control group care |
| Balaban 2008 |
Discharge planning lead: discharge planner registered nurse Timing of discharge plan: enrolled at admission to hospital Education:a patient discharge form for the patient that included information about the patient's health problem/diagnosis, medications, and follow‐up care Implementation of the discharge plan: discharge form was sent electronically to the primary care team to become part of the permanent medical records. |
A discharge plan to improve communication between inpatient and outpatient care teams abd to reconnect patients who lived at home with their primary care team, using a structure‐process‐outcome approach. The intervention was structured for a culturally diverse population. | Telephone call: the day after discharge from hospital, from the primary care nurse | No communication between hospital and primary care nurse, handwritten discharge instruction in English, communication with hospital and primary care physician as required. |
| Bolas 2004 |
Discharge planning lead: one full‐time clinical pharmacist clinical pharmacy service Timing of discharge plan: within 48 hours of admission to hospital Education: patient counselling to explain changes to medication Implementation of the discharge plan: daily contact with the patient to explain changes to treatement, medication history, personalised medication record, discharge letter outlining drug history and changes to medication during hospital and variances to discharge prescription. This was faxed to GP and community pharmacist. Personalised medicine card, discharge counselling, labelling of dispensed medications under the same headings for follow‐up. |
A hospital based community liaison pharmacist to improve the management of medicines and communication between secondary and primary care during transition from secondary to primary care. | Medicines help line | Standard clinical pharmacy service that did not include discharge counselling |
| Bonnetti 2018 |
Discharge planning lead: pharmacist‐led medication counselling Timing of discharge plan: recruited when admitted to hospital, review of discharge medications Education: verbal counseling was delivered by the pharmacists to patients or their caregivers, which included explanations about the indications, benefits, therapeutic targets, doses, dosing schedule, routes, storage, length of therapy, refill pharmacy, and possible ADEs of each prescribed drug. Implementation of the discharge plan: All pharmacist interventions followed a structured format. |
A pharmacist led review of medicines to improve communication about medicines during transition from hospital. | Patients were contacted by telephone three and 15 days post‐discharge to reinforce the previous counseling session. | Standard care from pharmacists and other healthcare providers |
| Cajanding 2017 |
Discharge planning lead: cardiovascular nurse practitioner led structured discharge plan Timing of patient involvement with the discharge plan: the second day of a hospital admission Education: individualized lecture type discussion, provision of feedback, integrative problem solving, goal setting, and action planning at 3 consecutive daily sessions lasting between 30 to 45 minutes Implementation of the discharge plan: a structured programme based from the guidelines set by the American Heart Association, the National Heart Foundation of Australia, and the Philippine Heart Association. |
A nurse led structured discharge programme to improve the quality of care and support the transition from hospital to home | Telephone at 3 and 15 days for the intervention group | Usual care based on the Philippine Heart Association clinical practice guidelines |
| Eggink 2010 |
Discharge planning lead: clinical pharmicist Timing of patient involvement with the discharge plan: at discharge Education: none Implementation of the discharge plan: verbal and written information about (side) effects of, and changes in, their in hospital drug therapy from a clinical pharmacist upon hospital discharge and the discharge medication list was faxed to the community pharmacist, a copy was provide to the patient to give to the GP. |
A multifaceted clinical pharmacist discharge service on the number of medications discrepancies after discharge, recruited participants had 5 + medicines prescribed | Not reported | Usual care |
| Evans 1993 |
Discharge planning lead: not clear Timing of patient involvement with the discharge plan: recruited patients screened at admission for risk of adverse hospital outcome and to minimise inappropriate referrals to discharge planning; discharge planning implemented on day 3 of hospital admission Education: not reported Implementation of the discharge plan: referred to a social worker, assessment of support systems, living situation, finances and areas of need. Plans were implemented with measurable goals. |
General discharge plan | Not reported | Could be referred for discharge planning, usually on day 9 of admission |
| Farris 2014 |
Discharge planning lead: pharmacist case manager Timing of patient involvement with the discharge plan: day 2 or 3 of admission Education: medication counselling to improve medication adherence, every 2 to 3 days, and discharge counselling Implementation of the discharge plan: a discharge medication list and counselling on goals of treatment, medication and barriers to adherence. Primary care provider and community pharmacist received a copy of the discharge plan within 24 hours of discharge and usually within 6 hours, it included the discharge medication list, plans for dosage adjustments and monitoring, recommendations for preventing adverse drug events, with patient specific concerns such as adherence or cost issues highlighted. |
To improve medication related outcomes during transitions of care | Telephone call 3 to 5 days post‐discharge | Usual care was medication reconciliation at admission according to hospital policy, nurse discharge counseling and a discharge medication list for patients. The usual care discharge summary was transcribed and received in the mail by the primary |
| Gillespie 2009 |
Discharge planning lead: clinical pharmicists Timing of involvement with the discharge plan: at admission Education: education provided during the hospital admission, a review of medicines and discharge counselling Implementation of discharge plan: medicine review, patient provided with a copy of the discharge letter. The pharmacist provided a comprehensive account of all changes in drug therapy during the hospital stay, including the rationale behind medication decisions, monitoring needs, and expected therapeutic goals. Drug related problems were listed with suggested actions. The physician responsible for the patient on the ward was required to approve the contents of the pharmacist’s discharge letter before it was sent to the patient’s general practitioner with the original discharge letter. The pharmacists’ discharge letters were not given to the patients. |
To reduce drug related problems, increase patient safety and reduce use of hospital care in people aged 80 years and older | Telephone call 2 months post‐discharge to assess the management of medicines | Standard care from nurse or physician, pharmacist not involved |
| Goldman 2014 |
Discharge planning lead: registered nurse, included native Spanish and Chinese speakers Timing of involvement with the discharge plan: patients who had been admitted in the previous 24 hours were seen by the discharge planning registered nurse Education: disease‐specific patient education that included symptom recognition, medication reconciliation and strategies to navigate the health system. Motivational interviewing techniques and coaching to promote patient engagement. A study RN supplemented verbal instructions with language‐concordant written materials (30). A study RN reinforced teaching using the “teach‐back” method to ensure comprehension (31) Implementation of discharge plan: the discharge planning study registered nurse met with the patient and contacted the patients’ primary care providers to supply the inpatient physicians’ contact information. |
A discharge planning nurse led intervention to facilitate the transition from hospital to home | Study nurse practitioners visited patients within 24 hours of discharge, and called patients on days 1 to 3 and 6 to 10 after discharge. | The bedside RN's review of the discharge instructions, received by all patients. If requested by the medical team, the hospital pharmacy provided a 10 day medication supply and a social worker assisted with discharge. The admitting team was responsible for liaising with the patients' PC |
| Harrison 2002 |
Discharge planning lead: nurse led Timing of involvement with the discharge plan: within 24 hours of Education: a structured evidence based protocol for counselling and education to support heart failure self‐management Implementation of discharge plan: comprehensive discharge plan, hospital and community nurse liaison, standard discharge planning + a comprehensive program that added support to improve the transfer from hospital to home. Hospital and community nurses met to focus on the ‘outreach’ from the hospital and ‘in‐reach’ from the community during the transition. An inter‐sectoral continuity of care framework was used to identify gaps to specifically address 3 major aspects of a hospital‐to‐home transition: (1) supportive care for self‐management; (2) linkages between hospital and home nurses and patients; and (3) the balance of care between the patient and family and professional providers |
A nurse led discharge plan to improve the transition between hospital settings. | Telephone call within 24 hours of discharge | Usual home care visits, available to intervention group |
| Hendriksen 1990 |
Discharge planning lead: project nurse Timing of involvement with the discharge plan: at the time of admission Education: health condition and discharge arrangements Implementation of the discharge plan: patients had daily contact with the project nurse who discussed their illness with them and discharge arrangements; liaison between hospital and primary care staff. |
A co‐ordinated transfer from hospital to home for older people. | Project nurse, a maximum of two visits after discharge | Usual care |
| Jack 2009 |
Discharge planning lead: nurse discharge advocate (DA) Timing of involvement with the discharge plan: Education: the DA used scripts from the training manual to review the contents of an after hospital care plan with the patient. Implementation of the discharge plan: with information collected from the hospital team and the participant, the DA created the after‐hospital care plan (AHCP), which contained medical provider contact information, dates for appointments and tests, an appointment calendar, a colour‐coded medication schedule, a list of tests with pending results at discharge, an illustrated description of the discharge diagnosis, and information about what to do if a problem arises. Information for the AHCP was manually entered into a Microsoft Word template, printed, and spiral‐bound to produce an individualised, colour booklet. On the day of discharge the AHCP and discharge summary were faxed to the primary care provider. |
Reengineered hospital discharge to minimize hospital utilisation after discharge. | A clinical pharmacist telephoned the participants 2‐4 days after the index discharge to reinforce the discharge plan by using a scripted interview. The pharmacist had access to the AHCP and hospital discharge summary and, over several days, made at least 3 attempts to reach each participant. The pharmacist asked participants to bring their medications to the telephone to review them and address medication‐related problems; the pharmacist communicated these issues to the PCP or DA | Usual care. |
| Kennedy 1987 |
Discharge planning lead: gerontology clinical nurse specialist (GCNS) Timing of involvement with the discharge plan: during the hospital admission Education: focused on explaining and clarifying the discharge plan Implementation of the discharge plan: a comprehensive discharge planning protocol (CDPP) was developed for use by the Gerontological Clinical Nurse Specialist (GCNS). Components of the assessment included: health status, orientation level, knowledge and perception of health status, resource use pattern, functional status, skill level, motivation level, and sociodemographic data. The patient's level of dependency was measured using the Long‐Term Care Information System (LTCIS). The GCNS met with the patient and family, physician, and other health care providers to identify resources and support networks for the patient postdischarge. A summary of the assessment information and potential care needs were entered in the progress notes of the patient's chart.The GCNS assisted in the coordination of services. |
A comprehensive discharge planning protocol to improve the health delivered to older people in hospital. | One follow‐up visit to assess the arrangements and care delivered. | Discharge arranged by the primary nurse. |
| Kripalani 2012 |
Discharge planning lead: a pharmacist TIming of patient involvement with the discharge plan: at enrolment to the study during a patients admission to hospital Education: one or two counselling sessions to the patient by the pharmacist, that accounted for the patient's health literacy and aimed to support adherence and minimize adverse effects. Pharmacists used 'teach‐back' to confirm understanding. Implementation of the discharge plan: pharmacist assisted medication reconciliation, tailored inpatient counselling, provision of low‐literacy adherence aids. The pharmacists communicated with the treating physicians to resolve any clinically relevant, unintentional medication discrepancies. |
A tailored intervention to reduce medication errors at and after hospital discharge. | Telephone follow‐up after discharge by a research coordinator, follow‐up call by a pharmacist to address any issues in collaboration with the treating inpatient and outpatient physicians. | Medicine reconciliation and discharge counselling |
| Lainscak 2013 |
Discharge planning lead: a discharge co‐ordinator Timing of patient involvement with the discharge plan: within 48 hours of admission to hospital Education: yes Implementation of the discharge plan: the discharge coordinator assessed the patient situation and home care needs to identify any problems and specific needs. Patients and caregivers were actively involved in the discharge planning process, which was communicated and discussed with community care/home care nurse, general practitioner, social care worker, physiotherapist, and other providers of home services as appropriate to provide continuity of care and care coordination across different levels of health care. |
To coordinate discharge from hospital to post‐discharge care to reduce hospitalizations. | Discharge coordinator called the patient 48 hours after discharge to check adjustment to home environment and additional needs, phone calls continued up to 7 to 10 days after discharge when a home visit was scheduled. | Usual care, routine patient education with written and verbal information about COPD, supervise inhaler use, respiratory physiotherapy as indicated, and disease related communication between medical staff with patients and their caregivers |
| Laramee 2003 |
Discharge planning lead: heart failure nurse case manager Timing of patient involvement with the discharge plan: during admission Education: a 15 page booked on heart failure to support self‐management. Individualised and family education. Implementation of the discharge plan: early discharge planning and coordination of care; facilitated communication between the hospital team and the patient, involved the patient and family in developing a care plan; review and monitoring of medicines and appropriate recommendations. |
Hospital based nurse led case management to co‐ordinate care and reduce hospital utilization. | 12 weeks of telephone follow‐up | Usual care |
| Legrain 2011 |
Discharge planning lead: a dedicated geriatrician Timing of patient involvement with the discharge plan: during admission Education: education on self‐management of disease Implementation of the discharge plan: comprehensive chronic medication review according to geriatric prescribing principles, and detailed transition‐of care‐communication with outpatient health professionals. |
To co‐ordinate a patient centred mult‐modal comprehensive discharge plan for older people to reduce preventable readmission, depression and protein‐energy malnutrition. | Not reported | Usual care in an acute geriatrician unit |
| Lin 2009 |
Discharge planning lead: nurse led Timing of patient involvement with the discharge plan: during the hospital admission Education: not reported Implementation of the discharge plan: structured assessment of discharge planning needs, systematic individualised nursing instruction based on the patient’s individual needs, monitoring services and coordinated resources and arranging of referral placements for each patient. |
To improve discharge planning to meet care needs after discharge for older people admitted to hospital with a hip fracture. | Two home visits post‐discharge to provide support and consultation | Unstructured discharge instructions without following a standardised procedure |
| Lindpainter 2013 |
Discharge planning lead: nurse Timing of patient involvement with the discharge plan: during admission Education: yes Implementation of the discharge plan: included discharge diagnoses, medication, and plans for follow‐up and home care sent on the day of discharge by to the primary care physician and the local visiting nurse organization. This discharge fax supplemented the hospital discharge summary generated as usual by the staff physician in both the intervention and control groups. |
To co‐ordinate care to reduce adverse events and cost | Telephone access via a pager and home visit if required | Standard discharge fax to primary care |
| Lisby 2019 |
Discharge planning lead: nurse Timing of patient involvement with the discharge plan: Education: included assessment of patients' understanding of their discharge recommendations that included medicines Implementation of the discharge plan: an assessment of the patient’s overall situation and requirement for additional healthcare and help, a review of medicines, their comprehension of discharge recommendations, a simple discharge letter targeting the individual patient’s health literacy and a follow‐up telephone call. |
To co‐ordinate care to increase post‐discharge safety and reduce readmissions. | Two week post‐discharge telephone call | Standard discharge letter provided to the primary care physician, the patient sometimes received a copy. |
| Moher 1992 |
Discharge planning lead: a nurse Timing of patient involvement with the discharge plan: shortly after admission to clinical unit. Education: not reported Implementation of the discharge plan: by a nurse co‐ordinator. |
To co‐ordinate and facilitate a discharge plan, tests and procedures, liaise with members of the clinical team and to collect and collate patient information. | Not included | Standard care |
| Naji 1999 |
Discharge planning lead: Psychiatrist Time of patient involvement with the discharge plan: ‐ Education: ‐ Implementation of the discharge plan: psychiatrist telephoned GP to discuss patient and make an appointment for the patient to see the GP within 1 week following discharge. A copy of the discharge summary was given to the patient to hand‐deliver to the GP and a copy was posted to the GP. |
To optimise communication between secondary and primary care at the time of discharge. | Not included | A standard discharge summary |
| Naughton 1994 |
Discharge planning lead: nurse Timing of discharge plan: at admission Education: yes Implementation of discharge plan: implemented at the time of admission; team meetings with the GEM and nurse specialist and physical therapist took place twice a week to discuss patients' medical condition, living situation, family and social supports, and patient and family's understanding of the patient's condition. The social worker was responsible for identifying and co‐ordinating community resources and ensuring the post‐discharge care was in place at the time of discharge and 2 weeks later. The nurse specialist co‐ordinated the transfer to home healthcare. Patients who did not have a primary care provider received outpatient care at the hospital. |
To build on geriatric management through a care plan that included co‐ordination of post‐discharge care. | Routine follow‐up that was not part of the discharge plan | Standard care |
| Naylor 1994 |
Discharge planning lead: nurse Timing of discharge plan: at admission Education: yes Implementation of discharge plan: 1) comprehensive initial and ongoing assessment of the discharge planning needs of the elderly patient and his or her caregiver; 2) development of a discharge plan in collaboration with the patient, caregiver, physician, primary nurse, and other members of the health care team; 3) validation of patient and caregiver education; 4) coordination of the discharge plan throughout the patient's hospitalization and through 2 weeks after discharge; 5) interdisciplinary communication regarding discharge status; and 6) ongoing evaluation of the effectiveness of the discharge plan. |
Timely discharge and facilitate post‐discharge care. | Telephone advise was available for up to two weeks after discharge and the nurse initiated two telephone calls during the first 2 weeks after discharge. | Routine discharge plan that was used for all patients |
| Nazareth 2001 |
Discharge planning lead: hospital and community pharmacists offered an integrated discharge plan. Timing of discharge plan: not clear. Education: the hospital pharmacist provided patients with information on their medicines and liaised with their carers and community professionals when appropriate, counselled patients and their caregivers on the purpose of the medicines, doses and how to dispose of excess medicines and provided carers and health professionals with a copy of the discharge plan. Implementation of discharge plan: Medication review and counselling, the hospital pharmacist assessed the patient's medication and the ability of the patient to manage their medication, provided medicine aids such as large print and special labels. |
Co‐ordination by hospital and community pharmacists to improve care of older people who are prescribed four or more drugs and optimise communication between primary and secondary care professionals | A pharmacist visited the patient at home two weeks after discharge from hospital to review medicines. | Standard discharge letter with diagnosis, investigations and medication, this did not include a review of medicines or a post‐discharge follow‐up visit. |
| Nguyen 2018 |
Discharge planning lead: hospital pharmacist TIming of discharge plan: one week before discharge Education: advise on their condition (acute coronary syndrome), risk factors, prevention; experience of medicines, medication aids, teaching back and correcting misunderstandings. Implementation of the discharge plan: Medication review and counselling, a multi‐faceted intervention of two counselling sessions to assess patients knowledge of their condition (acute coronary syndrome). |
A multi‐faceted intervention to enhance medication adherence, and reduce mortality and hospital readmission | Two weeks after discharge a 30 minute telephone call by a pharmacist to assess general and medication issues, provide tailored advice, teaching back and correcting misunderstanding | Standard care |
| Parfrey 1994 |
Discharge planning lead: member of the multi‐disciplinary team Timing of discharge plan: at admission Education: Implementaiton of the discharge plan: a 1‐page, 65‐item questionnaire was used to identify patients for early discharge planning. |
Early identification of patients for dicharge planning to reduce hospital length of stay | No | Standard discharge arrangements |
| Preen 2005 |
Discharge planning lead: multidisciplinary discharge care planning with primary care providers Timing of discharge plan: 24‐48 hours prior to discharge Education: patients were involved in identifying problems and goals Implementation of the discharge plan: problems and goals identified with the patient and carer, community service providers were identified who met patient needs and who were accessible.The discharge plan was faxed to the GP and all service providers identified on the care plan. |
A discharge care planning model to provide quality discharge arrangements and facilitate continuity of care and communication between the hospital and primary care physician | GP scheduled a consultation (within 7 d postdischarge) for patient review | Standard care that included a discharge summary provide to the patients and GP |
| Rich 1993 |
Discharge planning lead: cardiovascular specialist nurse Timing of discharge plan: early in the hospital admission Education: education about heart failure, treatment plan, diet and medicines using a 5 page guide Implementation of the discharge plan: review of medicines with recommendations to support compliance and reduce adverse effects, early discharge planning, review by social worker and home care team. The discharge plan was sent to the home care division. |
To facilitate discharge planning and ease the transition from hospital to home | Home care visited the patient at home within 48 hours of discharge and two more times during the first week, and then at regular intervals. | Standard care, without the education materials or formal medication review |
| Rich 1995 |
Discharge planning lead: cardiovascular specialist nurse Timing of discharge plan: early in the hospital admission Education: education about heart failure, treatment plan, diet and medicines using a 5 page guide Implementation of the discharge plan: review of medicines with recommendations to support compliance and reduce adverse effects, early discharge planning, review by social worker and home care team. The discharge plan was sent to the home care division. |
Reduce the risk of readmission | Home care visited the patient at home within 48 hours of discharge and two more times during the first week, and then at regular intervals. | Standard care, without the education materials or formal medication review |
| Shaw 2000 |
Discharge planning lead: hospital pharmacist Timing of discharge plan: during hospital admission Education: patient knowledge of illness and medicines was assessed by a questionnaire, and information was provided Implementation of the discharge plan: Medication review and counselling, a checklist was used to identify needs, details of the treatment plan were provided and provided to the patient's community pharmacist |
To identify medication problems experienced by patients | Domiciliary visits at 1, 4 and 12 weeks to assess knowledge and continuing care needs. | Standard care |
| Sulch 2000 |
Discharge planning lead: senior nurse with multi‐disciplinary team Timing of the discharge plan: day 5 to 6 of hospital admission Education: patient and carer education about the care plan and rehabilitation process, medicines, prognosis and related health problems Implementation of the discharge plan: discharge plan was revised during the hospital stay, the plan included discharge options and a date of discharge |
An integrated care pathway to reduce hospital length of stay in people with a stroke and having specialist rehabilitation | Routine follow‐up that was not part of the discharge plan | Standard multi‐disciplinary care |
| Weinberger 1996 |
Discharge planning lead: primary care nurse Timing of discharge plan: three days before discharge Education: patients were provided with educational material. Implementation of the discharge plan: assessment of post‐discharge needs, listed medical problems, assigned the patient to a primary care physician. |
Targetted people with diabetes, chronic obstructive pulmonary disease or heart failure who were at risk of readmission. Aimed to reduce readmission by strengthening the planning of post‐discharge care | Primary nurse telephoned the patient 2 days after discharge, patient given an appointment to attend the primary care clinic within one week of discharge. | Standard care, did not have access to primary care nurse and did not receive supplemental education or assessment of needs beyond usual care. |
The discharge plan was implemented at varying times during a participant's stay in hospital, from admission to three days prior to discharge. Of the 33 included trials, 15 followed up after discharge with a telephone call (Balaban 2008; Bolas 2004; Bonetti 2018; Cajanding 2017; Farris 2014; Gillespie 2009; Harrison 2002; Jack 2009; Kripalani 2012; Lainscak 2013; Laramee 2003; Lin 2009; Lindpaintner 2013; Nguyen 2018; Weinberger 1996), five offered a home visit (Hendriksen 1990; Kennedy 1987; Lindpaintner 2013; Naylor 1994; Shaw 2000), two scheduled primary care appointments (Preen 2005; Weinberger 1996), and13 did not report any form of follow‐up (Eggink 2010; Evans 1993; Goldman 2014; Legrain 2011; Lisby 2019; Moher 1992; Naji 1999; Naughton 1994;Nazareth 2001; Parfrey 1994; Rich 1993; Rich 1995; Sulch 2000).
In 17 trials discharge planning was nurse‐led (Balaban 2008; Cajanding 2017; Goldman 2014; Harrison 2002; Hendriksen 1990; Jack 2009; Kennedy 1987; Laramee 2003; Lin 2009; Lindpaintner 2013; Lisby 2019; Moher 1992; Naylor 1994; Rich 1993; Rich 1995; Sulch 2000; Weinberger 1996), in nine it was led by a pharmacist (Bolas 2004; Bonetti 2018; Eggink 2010; Farris 2014; Gillespie 2009; Kripalani 2012; Nazareth 2001; Nguyen 2018; Shaw 2000), in three a member of the multidisciplinary team or a discharge co‐ordinator (Lainscak 2013; Naughton 1994; Parfrey 1994), in one a psychiatrist (Naji 1999), a geriatrician (Legrain 2011) and for one the lead was not reported (Evans 1993).
Twenty‐four trials described the control group as receiving usual care with some discharge planning, that might be limited to a discharge letter, but without a formal link through a co‐ordinator to other departments and services, although other services were available on request from nursing or medical staff (Balaban 2008; Bonetti 2018; Cajanding 2017; Eggink 2010; Evans 1993; Gillespie 2009; Goldman 2014; Harrison 2002; Hendriksen 1990; Jack 2009; Laramee 2003; Legrain 2011; Lin 2009; Lisby 2019; Moher 1992; Naji 1999; Naylor 1994; Naughton 1994; Parfrey 1994; Preen 2005; Rich 1993; Rich 1995; Sulch 2000; Weinberger 1996). The control groups in nine trials that evaluated the effectiveness of a pharmacy discharge plan did not have access to a medicine review discharge plan by a pharmacist (Bolas 2004;Bonetti 2018; Eggink 2010; Farris 2014; Gillespie 2009; Kripalani 2012; Nazareth 2001; Nguyen 2018; Shaw 2000). Two trials considered the potential influence of language fluency (Balaban 2008; Goldman 2014), and two health literacy (Jack 2009; Kripalani 2012).
Excluded studies
The main reason for excluding studies was due to the intervention including the delivery of post‐discharge care, such as augmented home care, or being a small part of a multi‐component intervention (Characteristics of excluded studies).
Risk of bias in included studies
Risk of bias assessments are graphically displayed in Figure 2.
2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Twenty‐five trials reported adequate random sequence generation (Bolas 2004; Bonetti 2018; Cajanding 2017; Eggink 2010; Farris 2014; Gillespie 2009; Goldman 2014; Harrison 2002; Jack 2009; Kennedy 1987; Kripalani 2012; Lainscak 2013; Legrain 2011; Lindpaintner 2013; Lisby 2019; Moher 1992; Naji 1999; Naughton 1994; Nazareth 2001; Nguyen 2018; Rich 1993; Rich 1995; Shaw 2000; Sulch 2000; Weinberger 1996), this was unclear for the remaining trials. We assessed 20 trials as having low risk of allocation concealment (Bonetti 2018; Farris 2014; Gillespie 2009; Goldman 2014; Harrison 2002; Jack 2009; Kennedy 1987; Kripalani 2012; Lainscak 2013; Legrain 2011; Naji 1999; Naughton 1994; Nazareth 2001; Nguyen 2018; Parfrey 1994; Preen 2005; Rich 1995; Shaw 2000; Sulch 2000; Weinberger 1996), this was unclear for the remaining trials. We assessed two trials to be at unclear risk for differences in baseline characteristics (Balaban 2008; Laramee 2003), and two as unclear for differences in outcome measures at baseline (Bolas 2004; Laramee 2003), the remaining trials were assessed as low risk of bias for these domains.
Blinding
We assessed 25 trials as low risk of bias for the measurement of the primary outcomes (readmission and length of stay), as investigators used routinely‐collected data to measure these outcomes (Balaban 2008; Bolas 2004; Cajanding 2017; Eggink 2010; Evans 1993; Gillespie 2009; Goldman 2014; Harrison 2002; Hendriksen 1990; Jack 2009; Kennedy 1987; Kripalani 2012; Lainscak 2013; Laramee 2003; Legrain 2011; Moher 1992; Naji 1999; Naughton 1994; Naylor 1994; Nazareth 2001; Parfrey 1994; Rich 1993; Rich 1995; Sulch 2000; Weinberger 1996); one trial as high risk of bias as outcome data were collected by interview rather than through routine data collection (Lindpaintner 2013) The remaining seven trials had an unclear risk of bias for this criterion.
Incomplete outcome data
Four trials were assessed as high risk of bias for incomplete outcome data, range between 19% to 33% (Bolas 2004; Bonetti 2018; Cajanding 2017; Nguyen 2018), three trials as unclear risk of bias (Hendriksen 1990; Naji 1999; Shaw 2000), and the remaining trials as low risk of bias.
Selective reporting
The funnel plots (Figure 3; Figure 4) for hospital length of stay and readmission reflect the small number of underpowered studies included in the review.
3.

Funnel plot of the effect of discharge planning on hospital length of stay
4.

Funnel plot of the effect of discharge planning on unscheduled readmission rates, outcome, average follow‐up within 3 months of discharge from hospital.
Other potential sources of bias
One study (Legrain 2011) used the Zelen patient preference method for randomisation, 380 individuals were randomised but not included in the study as they did not provide consent; and one study reported that after one year of recruitment, less than half of the required study sample was included and the study was terminated (Lisby 2019).
Fidelity of the intervention delivered.
A small number of studies reported difficulties with the implementation of discharge planning. In one trial the authors reported that the delivery of the intervention by two pharmacy case managers varied (Farris 2014), and Cajanding 2017 reported that 8/107 (7.5%) in the intervention group did not complete the intervention.
Effects of interventions
See: Table 1
Hospital length of stay
People admitted to hospital with a medical condition
There was a small reduction in the initial hospital length of stay for those allocated to discharge planning in trials that recruited older people following a medical admission (mean difference (MD) − 0.73 days, 95% confidence interval (CI) − 1.33 to − 0.12; I 2 9%; 11 trials, 2113 participants; moderate‐certainty evidence) (Analysis 1.1).
1.1. Analysis.

Comparison 1: Effect of discharge planning on hospital length of stay, Outcome 1: Hospital length of stay ‐ older people with a medical condition
Following surgery
Discharge planning may lead to a small reduction in length of stay in participants who were recovering from surgery (mean difference (MD) ‐ 0.06/ a day, 95% CI − 1.23 to 1.11; I 2 0%; 2 trials, 184 participants; low‐certainty evidence) (Lin 2009; Naylor 1994) (Analysis 1.2).
1.2. Analysis.

Comparison 1: Effect of discharge planning on hospital length of stay, Outcome 2: Hospital length of stay ‐ older people following surgery
Studies recruiting people with medical condition or recovering from surgery
Three studies recruited a mix of participants recovering from surgery and those with a medical condition, two reported a reduction of less than one day in the groups allocated to discharge planning (Evans 1993; Parfrey 1994) and one a reduction of just over three days (Hendriksen 1990) (Analysis 1.3) (low‐certainty evidence).
1.3. Analysis.
Comparison 1: Effect of discharge planning on hospital length of stay, Outcome 3: Hospital length of stay ‐ studies recruiting people with a mix of conditions
| Hospital length of stay ‐ studies recruiting people with a mix of conditions | |
| Study | Heading 1 |
| Evans 1993 | Initial hospital length of stay T: Mean number of days in hospital 11.9 (SD 12.7) N=417 C: Mean number of days in hospital 12.5 (SD 13.5) N=418 |
| Hendriksen 1990 | Initial hospital length of stay T: 11 N=135 C: 14.3 N=138 |
| Parfrey 1994 | Recruited from two hospitals, reported a median difference for one hospital: − 0.80 days, P = 0.03; Intervention N=421; Control N=420 |
Readmission to hospital
People admitted to hospital with a medical condition
For older people with a medical condition, discharge planning led to a relative reduction in readmissions to hospital (average follow‐up within three months;risk ratio (RR) 0.89, 95% CI 0.81 to 0.97; 17 trials, I2 15%; 5126 participants; moderate‐certainty evidence).
People admitted to hospital for surgery
Two studies that recruited people recovering from surgery reported data on readmissions (low‐certainty evidence), one reported a 3% difference in readmission rates (95% CI − 7% to 13%; 134 participants) (Naylor 1994) and a second reported little or no difference (Lin 2009) (Analysis 2.2).
2.2. Analysis.
Comparison 2: Effect of discharge planning on unscheduled readmission rates, Outcome 2: Hospital readmission rates at various follow‐up times
| Hospital readmission rates at various follow‐up times | ||
| Study | Results | Notes |
| Participants with a medical condition | ||
| Bonetti 2018 | Mean hospital readmissions T= 4 (7.8) N=51, C= 7 (13.2) (N=53) |
Follow‐up: 30 days |
| Farris 2014 | At 30 d: T= 47/281 (17%), C = 43/294 (15%) Difference 2%; 95% CI − 0.04% to 0.08% At 90 d: T= 49/281 (17%), C = 47/294 (16%) Difference 1%; 95% CI − 5% to 8% |
— |
| Gillespie 2009 | At 12 months: T= 106/182 (58.2%), C = 110/186 (59.1%) Difference − 0.9%, 95% CI − 10.9% to 9.1% |
— |
| Goldman 2014 | At 30 d: T= 50/347 (14%), C = 47/351 (13%) Difference 1%; 95% CI − 4% to 6% At 90 d: I = 89/347 (26%), C = 77/351 (22%) Difference 3.7%; 95% CI − 2.6% to 10% |
Data provided by the trialists |
| Kennedy 1987 | At 1 week:
T= 2/38 (5%), C = 8/40 (20%)
Difference − 15%; 95% CI − 29% to − 0.4% At 8 weeks: I = 11/39 (28%), C = 14/40 (35%) Difference − 7%; 95% CI − 27.2% to 13.6% |
— |
| Lainscak 2013 | At 90 d: COPD− related T= 14/118 (12%), C = 33/135 (24%) Difference 12%; 95% CI 3% to 22% All‐cause readmission T = 25/118 (21%), C = 43/135 (32%) Difference 11%; 95% CI − 0.3% to 21% |
Data provided by the trialists; data also available for 30− and 180− d |
| Laramee 2003 | At 90 d:
T = 49/131 (37%), C = 46/125 (37%), P > 0.99 Readmission days: T= 6.9 (SD 6.5), C = 9.5 (SD 9.8) |
— |
| Lindpaintner 2013 | Similar readmission rate to hospital for both groups at 5 and 30 days | As reported by the authors; no further data reported T = 30, C = 30 |
| Lisby 2019 | At 30 d: T = 22/101 (22%), C = 19/99 (19%) Difference 3%; 95% CI ‐8.2% to 14.13 Total readmissions: T = 0.28 (SD 0.67); C = 0.26 (SD 0.63) |
Number of participants who were admitted at least once in each group Authors also report days to first readmission, and preventable first readmission Ascertained by chart review T = 101, C = 99 |
| Moher 1992 | At 2 weeks: T = 22/136 (16%), C = 18/131 (14%) Difference 2%; 95% CI − 6% to 11%, P = 0.58 | — |
| Naylor 1994 | Within 45‐90 d: T = 11/72 (15%), C = 11/70 (16%) Difference 1%; 95% CI − 8% to 12% | Authors also report readmission data for 2‐6 weeks follow up |
| Nazareth 2001 | At 90 d:
T = 64/164 (39%), C = 69/176 (39.2%)
Difference 0.18; 95% CI − 10.6% to 10.2% At 180 d: T = 38/136 (27.9%), C = 43/151 (28.4%) Difference 0.54; 95% CI − 11 to 9.9% |
— |
| Nguyen 2018 | Total number of participants readmitted T = 7/58 (12%), C = 6/68 (9%) Difference 3%, 95% CI ‐7.99 to 14.81 |
Follow‐up: 90 days |
| Weinberger 1996 | Number of readmissions per month
T = 0.19 (+ 0.4) (n = 695), C = 0.14 (+ 0.2), P = 0.005 (n = 701) At 6 months: T = 49%, C = 44%, P = 0.06 Treatment group readmitted 'sooner' (P = 0.07) |
Non‐parametric test used to calculate P values for monthly readmissions |
| Participants with medical or surgical condition | ||
| Evans 1993 | At 4 weeks:
T = 103/417 (24%), C = 147/418 (35%)
Difference − 10.5%; 95% CI − 16.6% to − 4.3%, P < 0.001 At 9 months: T = 229/417 (55%), C = 254/418 (61%) Difference − 5.8%; 95% CI −12.5% to 0.84%, P = 0.08 |
— |
| Participants recruited following surgery | ||
| Lin 2009 | Within 3 months: T=2/26 (7.7%), C=2/24 (8.3%) |
‐ |
| Naylor 1994 | Within 6 to 12 weeks: T = 7/68 (10%), C = 5/66 (7%) Difference 3%; 95% CI 7% to 13% | — |
| Participants with a mental health diagnosis | ||
| Naji 1999 | At 6 months: T = 33/168 (19.6%), C = 48/175 (27%) Difference 7.4%; 95% CI − 1.1% to 16.7% | Mean time to readmission T = 161 d, C = 153 d T: treatment; C: control; CI: confidence interval |
| Shaw 2000 | At 90 d: T = 5/51 (10%), C = 12/46 (26%) | |
People admitted to hospital with a mental health diagnosis
Two studies that recruited participants admitted to mental health facilities reported data on readmissions (low‐certainty evidence), one reported a difference of 7% (95% CI − 1% to 17%; 343 participants) (Naji 1999) and a second a reduction in readmission to hospital (T = 5/51 (10%), C = 12/46 (26%); 97 participants (Shaw 2000) (Analysis 2.2).
Studies recruiting people with medical condition or recovering from surgery
One trial (Evans 1993), reported a reduction in readmission rate to hospital for those receiving discharge planning (difference − 10.5%, 95% CI − 16.6% to − 4.3%) at four weeks follow‐up, but not at nine months (difference − 5.8%, 95% CI − 12.5% to 0.84%; P = 0.08; Analysis 2.2) (low ‐certainty evidence).
Patient health status
Mortality reported in studies that recruited people admitted to hospital with a medical condition
For older people with a medical condition (usually heart failure) it is uncertain if discharge planning has an effect on mortality at three‐ to nine‐month follow‐up (RR 1.05, 95% CI 0.85 to 1.29; I 2 0%; ; 8 trials, 2721 participants; moderate‐certainty evidence) (Analysis 3.1); (Goldman 2014; Lainscak 2013; Laramee 2003; Legrain 2011; Nazareth 2001; Nguyen 2018; Rich 1995; Sulch 2000).
3.1. Analysis.

Comparison 3: Effect of discharge planning on health status, Outcome 1: Mortality at 3 to 9 months
Mortality reported in studies that recruited people with medical condition or recovering from surgery
One study reported data for mortality at nine‐month follow‐up (treatment: 66/417 (15.8%), control: 67/418 (16%) (low‐certainty evidence) (Evans 1993) Analysis 3.2).
3.2. Analysis.
Comparison 3: Effect of discharge planning on health status, Outcome 2: Mortality for trials recruiting participants with a medical condition and those recovering from surgery
| Mortality for trials recruiting participants with a medical condition and those recovering from surgery | ||
| Study | Mortality at 9 months | Notes |
| Evans 1993 | T = 66/417 (16%) C = 67/418 (16%) | — |
Health status and quality of life reported in studies that recruited people admitted to hospital with a medical condition
We are uncertain whether discharge planning improves patient reported health status or quality of life (12 studies, 2927 participants when reported; low‐certainty evidence) due to variability among the trials and the range of measures used to assess health status (Harrison 2002; Kennedy 1987 Preen 2005; Weinberger 1996; Sulch 2000; Lainscak 2013; Lindpaintner 2013; Nguyen 2018; Lisby 2019; Nazareth 2001; Cajanding 2017; Rich 1995) (Analysis 3.3).
3.3. Analysis.
Comparison 3: Effect of discharge planning on health status, Outcome 3: Patient‐reported outcomes: a medical condition
| Patient‐reported outcomes: a medical condition | ||
| Study | Patient health outcomes | Notes |
| Patients with a medical condition | ||
| Cajanding 2017 |
MLHFQ Mean difference (C ‐ T) 8.59 (SD 2.29), 95% CI 4.02 to 13.16 CSE Mean difference (C ‐ T) ‐5.61 (SD 1.13), 95% CI ‐7.87 to ‐3.36 |
Minnesota Living With Heart Failure Questionnaire (MLHFQ): a lower score indicates less disability from symptoms Cardiac Self‐Efficacy Questionnaire (CSE): higher scores represent higher self‐confidence Follow‐up: 30 days As reported by the authors, mean difference at follow‐up T = 75, C = 68 C: control; T: treatment; SD: standard deviation |
| Harrison 2002 |
SF‐36 Baseline Physical component T = 28.63 (SD 9.46) N = 78 C = 28.35 (SD 9.11) N = 78 Mental component T = 50.49 (SD 12.45) N = 78 C = 49.81 (SD 11.36) N = 78 At 12 weeks Physical component T = 32.05 (SD 11.81) N = 77 C = 28.31 (SD 10.0) N = 74 Mental component T = 53.94 (SD 12.32) N = 78 C = 51.03 (SD 11.51) N = 78 MLHFQ At 12 week follow‐up (See table 4) n, % Worse: T = 6/79 (8), C = 22/76 (29) Same: T = 7/79 (9), C = 10/76 (13) Better: T = 65/79 (83), C = 44/76 (58) |
SF‐36 a higher score indicates better health status MLHFQ: a lower score indicates less disability from symptoms T = 79, C = 76 (at 12 week follow‐up) |
| Kennedy 1987 | Long Term Care Information System (LTCIS) Health and functional status (also measures services required) | No data reported T = 39, C = 41 |
| Lainscak 2013 |
St. George’s Respiratory
Questionnaire (SGRQ) Change in score from 7 to 180 days after discharge T = 1.06 (IQR CI 8.43 to − 9.50), C = − 0.11 (IQR 8.12 to − 11.34) |
Complete data available for approximately half of the participants allocated to the intervention and comparison groups For the SGRQ, higher scores indicate more limitations; minimal clinically important difference estimated as 4 points. T = 63, C = 72 |
| Lisby 2019 |
VAS T = 60.4 (95% CI 55.4 to 65.5), N = 76; C = 60.2 (95% CI 55.1 to 65.4), N = 81. P = 0.96 |
Visual Analogue Scale (0‐100, higher scores represent better perceived health) Mean scores at 30 days post‐discharge; authors also report EQ‐5D scores for each item T = 76, C = 81 |
| Naylor 1994 | Data aggregated for both groups. Mean Enforced Social Dependency Scale increased from 19.6 to 26.3 P < 0.01 | Decline in functional status reported for all patients. Scale measured:
T = 72, C = 70 |
| Nazareth 2001 |
General well‐being questionnaire: 1 = ill health, 5 = good health
At 3 months:
T = 76, mean 2.4 (SD 0.7)
C = 73, mean 2.4 (SD 0.6) At 6 months: T = 62, mean 2.5 (SD 0.6) C = 61, mean 2.4 (SD 0.7) Mean difference 0.10; 95% CI − 0.14 to 0.34 |
T = 62, C = 61 (at 6 months follow‐up) |
| Nguyen 2018 | EQ‐5D‐3L T = median 0.000 (IQR 0.000 to 0.275), C = 0.234 (IQR 0.000 to 0.379) |
European Quality of Life Questionnaire – (EQ‐5D‐3L). Dimensions: mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has 3 levels: no problem, some problems, and extreme problems IQR: Interquartile range T = 79, C = 87 Follow‐up: 90 days Changes in quality of life from baseline at the first 3 months after discharge. Data as reported by the authors, no additional data available |
| Preen 2005 | SF‐12 Mental component score Predischarge score: T = 37.4 SD 5.4 C = 39.8 SD 6.1 7 d postdischarge: T = 42.4 SD 5.6 C = 40.9 SD 5.7 Physical component score Predischarge score: T = 27.8 SD 4.8 C = 28.3 SD 4.7 7 d postdischarge: T = 27.2 SD 4.5 C = 27.2 SD 4.1 |
Baseline N: T 91 C 98 Number at follow‐up not reported. |
| Rich 1995 |
Chronic Heart Failure Questionnaire Total score At baseline: T = 72.1 (15.6), C = 74.4 (16.3) At 90 d: T = 94.3 (21.3), C = 85.7 (19.0) Change score = 22.1 (20.8), P = 0.001 Dyspnoea At baseline: T = 9.0 (7.9), C = 8.1 (7.7) At 90 d: T = 15.8 (12.8), C = 11.9 (10.0) Change score 6.8 (7.9) Fatigue At baseline: T = 12.9 (5.3), C = 14.1 (5.6) At 90 d: T = 18.3 (6.3), C = 16.8 (5.5) Change score 5.4 (5.5) Emotional function At baseline: T = 31.9 (8.5), C = 33.3 (8.1) At 90 d: T = 37.4 (7.8), C = 35.2 (8.4) Change score 5.6 (7.1) Environmental mastery At baseline: T = 18.3 (5.8), C = 18.9 (4.8) At 90 d: T = 22.7 (4.9), C = 21.7 (4.6) Change score 4.4 (5.3) |
Treatment N = 67, Control N = 59 Chronic Heart Failure Questionnaire contains 20 questions that the patient is asked to rate on a scale 1 to 7 with a low score indicating poor quality of life |
| Sulch 2000 |
Barthel activities of daily living
Median scores At 4 weeks: T = 13, C = 11 At 12 weeks: T = 15, C = 17 At 26 weeks: T = 17, C = 17 Median change from 4 to 12 weeks: P < 0.01 Rankin score Median score At 4 weeks: T = 1, C = 1 At 12 weeks: T = 3, C = 3 At 26 weeks: T = 3, C = 3 Hospital anxiety and depression scale Anxiety Median scores At 4 weeks: T = 5, C = 5 At 12 weeks: T = 4, C = 4 At 26 weeks T = 4, C = 4 Depression Median scores At 4 weeks: T = 6, C = 5 At 12 weeks: T = 5, C = 5 At 26 weeks: T = 5, C = 5 EuroQol At 4 weeks: T = 41, C = 44 Median scores At 4 weeks: T = 41, C = 44 P = 0.10 At 12 weeks: T = 59, C = 65 P = 0.07 At 26 weeks: T = 63, C = 72 P < 0.005 |
The Barthel ADL Index covers activities of daily living; scores range from 0 to 20, with higher scores indicating better functioning. The Rankin scale assesses activities of daily living in people who have had a stroke; it contains 7 items with scores ranging from 0 to 6. Higher scores indicating more disability. The Hospital Anxiety and Depression Scale is a 14‐item Likert scale (0‐3); scores range from 0 to 21 for each subscale (anxiety and depression), with higher scores indicating more burden from symptoms. The EuroQol contains 5 items; higher scores indicate better self‐perceived health status. Baseline T = 76, C = 76 |
| Weinberger 1996 | At 1 month: no significant differences
P = 0.99 At 3 months: no significant differences P = 0.53 |
SF‐36 T = 695, C = 701 No data shown |
| Patient report outcomes following surgery | ||
| Lin 2009 |
OARS Multidimensional Functional Assessment Questionnaire (Chinese version) at 3 months follow‐up Mean (SD) T = 16.92 (1.41) C = 16.83 (1.71) |
9 components, each component scored 0 to 2 with a total score range 0‐18. T = 26, C = 24 |
| Naylor 1994 | No differences between groups reported | Decline in functional status reported for all patients. Scale measured:
T = 68, C = 66 |
| Patients with a medical or surgical condition | ||
| Evans 1993 | At 1 month: mean (SD) T = 85.3 (21.0) n = 417 C = 86.5 (21.0) n = 418 Difference − 1.2; 95% CI − 4.05 to 1.65 | Barthel score (scale 1 to 100) |
| Patients with a mental health diagnosis | ||
| Naji 1999 |
Hospital Anxiety Depression Scale
At 1 month after discharge, median (IQR) Anxiety T = 11.0 (6.0, 15.0), C = 10.0 (5.0, 14.0) Mann Whitney P = 0.413 Depression T = 9.5 (5.0, 13.3), C = 7.0 (3.0, 11.0) Mann Whitney P = 0.016 Behavioural and Symptom Identification Scale Relation to self/other T = 1.8 (1.2, 2.8), C = 1.7 (0.4, 2.7) Mann Whitney P = 0.10 Depression/anxiety T = 1.7 (0.8, 2.7), C = 1.5 (0.4, 2.4) Mann Whitney P = 0.46 Daily living/role functioning T = 2.0 (0.9, 2.8), C = 1.8 (0.8, 2.8) Mann Whitney P = 0.37 Impulsive/addictive behaviour T = 0.7 (0.3, 1.6), C = 0.7 (0.1, 1.5) Mann Whitney P = 0.89 Psychosis T = 0.5 (0.2, 0.8), C = 0.7 (0.2, 1.0) Mann Whitney P = 0.31 Total symptom score T = 1.4 (0.6, 2.1), C = 1.3 (0.5, 2.1) Mann Whitney P = 0.54 |
Number recruited: T=168; C=175 |
Health status and quality of life reported in studies that recruited people in hospital following surgery
We are uncertain whether discharge planning improves patient reported health status or quality of life (2 studies, 184 participants; low‐certainty evidence) (Lin 2009, Naylor 1994) (Analysis 3.3).
Health status and quality of life reported in studies that recruited people admitted to a mental health facility
One trial (Naji 1999) that recruited 343 participants admitted to a psychiatric unit reported little or no difference at one month post‐discharge for health status or psychological health (low‐certainty evidence) (Analysis 3.3).
Health status and quality of life reported in studies that recruited people with medical condition or recovering from surgery
There was little to no difference in mean scores between groups in the trial that recruited people with a medical condition and recovering from surgery (835 participants; low‐certainty evidence) (Evans 1993).
Satisfaction of patients, caregivers and healthcare professionals with discharge planning
Eight trials reported various aspects of satisfaction with discharge planning (low certainty evidence). Four trials (n = 2026) reported that discharge planning may lead to increased satisfaction with the discharge process or care received for patients with a medical diagnosis (lo‐ certainty evidence) (Cajanding 2017; Laramee 2003; Moher 1992; Weinberger 1996), and two trials reported similar scores between groups (Lisby 2019; Nazareth 2001) (Analysis 4.1); one trial (n = 60) reported similar scores for caregivers in each group (Lindpaintner 2013) (Analysis 4.1); one reported few differences between groups in the satisfaction scores for healthcare professionals (Lindpaintner 2013), and one trial that the intervention may improve the standard of discharge information (Bolas 2004).
4.1. Analysis.
Comparison 4: Effect of discharge planning on satisfaction with care process, Outcome 1: Satisfaction
| Satisfaction | ||
| Study | Satisfaction | Notes |
| Patient and care givers' satisfaction | ||
| Cajanding 2017 |
SF‐PSQ‐18 Mean difference (C ‐ T) ‐17.33 (SD 2.73), 95% CI ‐22.78 to ‐11.89 |
Short‐Form Patient Questionnaire (SF‐PSQ‐18): higher scores represent more satisfaction with medical care. Follow‐up: 30 days N: T = 75, C = 68 As reported by the authors, mean difference at follow‐up |
| Laramee 2003 | Mean hospital care: T = 4.2, C = 4.0, P = 0.003 Mean hospital discharge: T = 4.3, C = 4.0, P < 0.001 Mean care instructions: T = 4.0, C = 3.4, P < 0.001 Mean recovering at home: T = 4.4, C = 3.9, P < 0.001 Mean total score: T = 4.2, C = 3.8, P < 0.001 |
16‐item survey, 4 subscales (hospital care, hospital discharge, care instructions, and recovering at home). Items scored 1 to 5, higher scores reflect more satisfaction. N: T = 120, C = 100 Follow‐up: 3 months |
| Lindpaintner 2013 | Satisfaction with discharge process At 5 days (median and IQR) Patients: T = 1 (0), C = 1 (1‐2) Carers: T = 1 (0), C = 1 (1‐2) At 30 days Patients: T = 1 (1‐2), C = 1 (1‐2) Carers: T = 1 (1‐2), C = 2 (1‐3) |
4‐point Likert‐scale, lower scores indicate higher satisfaction N: T = 30, C = 30 Follow‐up: 5 and 30 days |
| Lisby 2019 | Overall satisfaction with discharge process: high or very high T = 48/74 (65%), C = 46/71 (65%) Difference 0%, 95% CI ‐15.24 to 15.18 |
Follow‐up: 30 days Single question, Likert‐scale |
| Moher 1992 | Satisfied with medical care: T = 89%, C = 62% Difference 27%; 95% CI 2% to 52%, P < 0.001 | "Please rate how satisfied you were with the care you received…" Subgroup of 40 patients, responses from 18 in the treatment group and 21 in the control group T = 136, C = 131 Follow‐up: 2 weeks |
| Nazareth 2001 | At 3 months:
T = 76, mean 3.3 (SD 0.6)
C = 73, mean 3.3 (SD 0.6) At 6 months: T = 62, mean 3.4 (SD 0.6) C = 61, mean 3.2 (SD 0.6) Mean difference 0.20; 95% CI − 0.56 to 0.96 |
Client Satisfaction Questionnaire score, 7 items (1 = dissatisfied, 4 = satisfied), higher scores indicate higher satisfaction. Follow‐up: 3 and 6 months |
| Weinberger 1996 | At 1 month:
Treatment group more satisfied, P < 0.001 At 6 months: Treatment group more satisfied, P < 0.001 Authors report differences were greatest for patients' perceptions of continuity of care and non‐financial access to medical care |
Patient Satisfaction Questionnaire, 11 domains with a 5‐point scale T = 695, C = 701 Follow‐up: 1 and 6 months |
| Professional's satisfaction | ||
| Bolas 2004 | Standard of information at discharge improved GPs: 57% agreed Community pharmacists: 95% agreed |
Response rate of 55% (GPs) and 56% (community pharmacists) No information provided about the survey |
| Lindpaintner 2013 | Satisfaction with discharge process At 5 days (median and IQR) Primary care physician: T = 1 (1‐2), C = 2 (1‐3) Visiting nurse: T = 1 (1‐2), C = 2 (1‐4) At 30 days (median and IQR) Primary care physician: T = 2 (1‐3), C = 1 (1‐2) |
Number of respondents ranged between 15 (visiting nurse) and 30 (PCP) 4‐point Likert scale, lower scores indicate higher satisfaction |
Healthcare resource use and cost
We downgraded the evidence to very low due to very serious inconsistency and imprecision.
People with a medical condition
It is uncertain whether there is any difference in hospital, primary or community care costs when discharge planning is implemented for patients with a medical condition (Farris 2014; Gillespie 2009; Goldman 2014; Jack 2009; Laramee 2003; Lisby 2019; Naughton 1994; Nazareth 2001; Rich 1995; Weinberger 1996) (Analysis 5.1; Analysis 5.2) (very low‐certainty evidence), or in the one trial that recruited people who had a surgical procedure (Naylor 1994).
5.1. Analysis.
Comparison 5: Effect of discharge planning on hospital resource use and cost, Outcome 1: Hospital cost
| Hospital cost | ||
| Study | Costs | Notes |
| Patients with a medical condition | ||
| Gillespie 2009 |
Total T: USD 12000; C: USD 12500 Mean difference: − USD 400 (− USD 4000 to USD 3200) Visits to ED T: USD 160; C: USD 260 Mean difference: − USD 100 (− USD 220 to − USD 10) Readmissions T: USD 12000; C: USD 12300 Mean difference: − USD 300 (− USD 3900 to USD 3300) |
Costs calculated for 2008 T = 182, C = 186 |
| Jack 2009 |
Emergency department visits T: USD 11,285 C: USD 21,389 Hospital visits T: USD 268,942 C: USD 412,544 Follow‐up primary care appointments* T: USD 12,617 C: USD 8906 Total cost difference between groups USD 149,995, Mean USD 412 per participant |
Follow‐up PCP appointments were given an estimated cost of USD 55, on the basis of costs from an average hospital follow‐up visit at Boston Medical Center * For 62% of 370 intervention participants and 44% of 368 usual care participants As reported by the authors, no further data available T = 373, C = 376 |
| Laramee 2003 |
Total inpatient and outpatient median costs
T = USD 15,979
C = USD 18,662 P = 0.14 |
The case manager (CM) kept a log during the first, middle and last 4 weeks of the recruitment period of how much time was spent with each patient during the 12‐week study period. Thus,
the average cost of the intervention was calculated based on an hourly wage (including benefits) of USD 33.93 for the CM. The average intervention cost per patient was USD 228.52, and the average time spent with each intervention patient was 6.7 h per 12 weeks. T = 141, C = 146 |
| Naughton 1994 | — | Number: T = 51, C = 60 Total cost of hospital care including breakdown of costs for laboratory, diagnostic imaging, pharmacy and rehabilitation services |
| Naylor 1994 |
Initial stay mean charges (USD):
T = 24,352 ± 15,920 (n = 72)
C = 23,810 ± 18,449 (n = 70)
Difference 542 (CI − 5121 to 6205) Medical readmission total charges in USD (CIs are in thousands): At 2 weeks: T = 68,754 C = 239,002 Difference = − 170,247 (CI − 253 to − 87) 2‐6 weeks: T = 52,384 C = 189,892 Difference = − 137,508 (CI − 210 to − 67) 6‐12 weeks: T = 471,456 C = 340,496 Difference = 130,960 (CI − 205 to 467) |
Charge data were used to calculate the cost of the initial hospitalisation Readmission costs were calculated using the mean charge per day of the index hospitalisations times the actual number of days of subsequent hospitalisations, as patients were readmitted to a variety of hospitals with a wide range of charges Total charges including readmission charges (first readmission only if multiple readmissions) T = 140, C = 136 |
| Rich 1995 |
Intervention cost USD 216 per patient Caregiver cost T = USD 1164, C = USD 828 Difference USD 336 Other medical care T = USD 1257, C = USD 1211 Difference USD 46 Readmission costs T = USD 2178, C = USD 3236 Difference − USD 1058 All costs T = USD 4815, C = USD 5275 Difference − USD 460 |
T = 142, C = 140 |
| Patients with a surgical condition | ||
| Naylor 1994 | Surgical initial stay mean charges (USD): T = 105,936 ± 52,356 (n = 68) C = 98,640 ± 52,331 (n = 66) Difference 7296 (CI − 5141 to 19,733) | Charge data were used to calculate the cost of the initial hospitalisation |
5.2. Analysis.
Comparison 5: Effect of discharge planning on hospital resource use and cost, Outcome 2: Primary and community care resource use and cost
| Primary and community care resource use and cost | ||
| Study | Use of services | Notes |
| Farris 2014 | Unscheduled office visits At 30 d T = 31/281 (11%), C = 32/294 (11%) Difference 0%; 95% CI − 5% to 5% At 90 d T = 42/281 (15%), C = 33/294 (11%) Difference 4%; 95% CI − 2 to 9% |
Results for Enhanced vs Control intervention (results for minimal intervention not reported) |
| Goldman 2014 | Primary care visits at 30 d T = 189/301 (62.8%), C = 186/316 (58.9%) Difference 4%; 95% CI − 3.7% to 11.5% |
— |
| Laramee 2003 | Visiting Nurse postdischarge: T = 70/141(50%), Control: 64/146 (44%) | — |
| Lisby 2019 | General practitioner contacts T = mean 3.6 (SD 2.3), C = mean 3.5 (SD 2.5) After‐hours visits T = mean 1.6 (SD 0.8), C = mean 1.9 (SD 1.7) |
Follow‐up: 30 days Ascertained by chart review T = 86, C = 93 SD: standard deviation |
| Nazareth 2001 | General practice attendance: At 3 months: T = 101/130 (77.7%) C = 108/144 (75%) Difference 2.7%; 95% CI − 7.4 to 12.7% At 6 months: T = 76/107 (71%) C = 82/116 (70.7%) Difference 0.3%; 95% CI −11.6 to 12.3% |
— |
| Weinberger 1996 | Median time from hospital discharge to the first visit:
Treatment 7 d
Control 13 d
P < 0.001 Visit at least one general medicine clinic in 6‐month follow up: Treatment 646/695 (93%) Control 540/701 (77%) Difference 16%; 95% CI 12.3% to 19.6%, P < 0.001 Mean number of visits to general medical clinic: Treatment 3.7 Control 2.2 P < 0.001 |
— |
Medication use
People admitted to hospital with a medical condition
Nine trials reported outcomes that related to medication. Six reported data on medication errors or problems identified at follow‐up (Analysis 6.1) (N=1,897 participants; very low‐certainty evidence). In Eggink 2010 68% in the control group had at least one discrepancy or medication error compared to 39% in the treatment group, Bonetti 2018 reported that those allocated to the control group had more medication problems (mean difference 3, 95% CI 1.8 to 4.2), Kripalani 2012 reported similar results for both groups in clinically important medication errors at 30 days (RR = 0.92, 95% CI 0.77 to 1.10), Bolas 2004 reported a higher rate of reconciliation of patient's own drugs with the discharge prescription, 90% compared to the 44% in the control group and Farris 2014 reported little or no difference between groups. Shaw 2000 reported on a range of problems, including difficulty about obtaining a prescription from the GP, finding a small difference favouring the intervention (mean difference 1, 95% CI 0.4 to 1.6).
6.1. Analysis.
Comparison 6: Effect of discharge planning on medication use, Outcome 1: Problems with medication after discharge from hospital
| Problems with medication after discharge from hospital | ||
| Study | Results | Notes |
| Bolas 2004 | Intervention group demonstrated a higher rate of reconciliation of patient's own drugs with the discharge prescription; 90% compared to the 44% in the control group | T = 119, C = 124 |
| Bonetti 2018 | Number of medication problems per participant T = M 1 (SD 1.5), C = M 4 (SD 4.2) Difference 3, 95% CI 1.8 to 4.2 |
Follow‐up: 30 days Reviewed by a pharmacist T = 51, C = 51 M: mean, SD: standard error |
| Eggink 2010 | Following a review of medication by a pharmacist, 68% in the control group had at least one discrepancy or medication error compared to 39% in the intervention group (RR 0.57; 95% CI 0.37 to 0.88). The percent of medications with a discrepancy or error in the intervention group was 6.1% in intervention group and 14.6% in the control group (RR = 0.42; 0.27 to 0.66). | T = 41, C = 44 Follow‐up: 6 weeks Reviewed by a pharmacist |
| Farris 2014 | Discharge T = 7.1 (SD 7.0), C = 6.1 (SD 6.6) 30 days post‐discharge T = 10.1 (SD 8.9), C = 9.6 (SD 9.5) P = 0.78 90 days post‐discharge T = 11.6 (SD 10.5), C = 11.1 (11.3) P = 0.94 |
T=307, C=309 at 30 day follow‐up As measured by the medication appropriateness index (MAI); summed MAI per participant Results for Enhanced v Control intervention (results for minimal intervention not reported) |
| Kripalani 2012 | Clinically important medication errors (total number of events; could be more than one per patient) At 30 d T = 370/423, M = 0.87 (SD 1.18) C = 407/428, M = 0.95 (SD 1.36) |
Follow‐up: 30 days |
| Shaw 2000 | Mean number of problems (SD) At 1 week: T = 2.0 (1.3), C = 2.5 (1.6) At 4 weeks: T = 1.9 (1.5), C = 2.9 (1.8) At 12 weeks: T = 1.4 (1.2), C = 2.4 (1.6) Difference 1, 95% CI 0.4 to 1.6 |
Problems included difficulty obtaining a prescription from the GP; insufficient knowledge about medication; non‐compliance T = 51, C = 46 |
Four trials reported data on adherence to medicines with very low‐certainty evidence (N= 648). Two trials reported little or no difference at follow‐up (low‐certainty evidence) (Bonetti 2018; Nazareth 2001), Nguyen 2018 reported little difference in medicine adherence at three months follow‐up in the discharge planning medicine review group (absolute difference 11%, 95% CI 11%, 95% ‐5.9 to 26.00), and Rich 1995 reported that 83% in the discharge plan medicine review group reported taking 80% or more of their prescribed medicines compared with 65% in the control group at 30 days after discharge (Analysis 6.2). Three trials assessed participants knowledge of medicines (Analysis 6.3).
6.2. Analysis.
Comparison 6: Effect of discharge planning on medication use, Outcome 2: Adherence to medicines
| Adherence to medicines | ||
| Study | Adherence to medicines | Notes |
| Bonetti 2018 |
Total MedTake T = mean 92.1 (SD 9.9), C = 58.5 (SD 31.9) ARMS T = mean 13 (SD 2), C = 15 (SD 4) |
Total MedTake: drug‐taking procedures for oral prescriptions; evaluates dosage, indications, food or water co‐ingestion, and regimens. Score corresponds to the percentage of correct actions (0%: zero adherence; 100%: total adherence) Adherence to Refils and Medications Scale (ARMS): medication adherence scale for patients with chronic medical conditions; 14 items, scores range between 12 and 48, higher scores reflect lower adherence. Self‐reported T: 49, C: 49 Follow‐up 30 days T: treatment; C: control; SD: standard deviation |
| Nazareth 2001 | At 3 months:
T = 79, mean 0.75 (SD 0.3), C = 72 mean 0.75 (SD 0.28) At 6 months: T = 60, mean 0.78 (SD 0.30), C = 58 mean 0.78 (SD 0.30) |
0 = none 1 = total/highest level |
| Nguyen 2018 | Participants assessed as adhering to their medication T = 53/70 (76%), C = 52/80 (65%) Absolute difference 11%, 95% ‐5.9 to 26.00) |
Follow‐up: 3 months Morisky Medication Adherence Scale (MMAS‐8): 8‐item questionnaire (items 1‐7 are dichotomous, last item is a Likert‐ale). for identification of barriers and behaviours associated with medication adherence. |
| Rich 1995 | Taking 80% or more of prescribed pills at 30 d after discharge T = 117/142 (82.5%), C = 91/140 (64.9%) |
— |
6.3. Analysis.
Comparison 6: Effect of discharge planning on medication use, Outcome 3: Knowledge about medicines
| Knowledge about medicines | ||
| Study | Knowledge | Notes |
| Bolas 2004 | Mean error rate in knowledge of drug therapy at 10‐14 d follow up Drug name T = 15%, C = 43%, P < 0.001 Drug dose T = 14%, C = 39%, P < 0.001 Frequency T = 15%, C = 39%, P < 0.001 (n for each group not reported) |
— |
| Nazareth 2001 | At 3 months:
T = 86, mean 0.69 (SD 0.33)
C = 83, mean 0.62 (SD 0.34) At 6 months: T = 65, mean 0.69 (SD 0.35) C = 68, mean 0.68 (SD 0.30) Mean difference 0.01; 95% CI − 0.12 to 0.13 |
0 = none 1 = total/highest level |
| Shaw 2000 | At 1 and 12 weeks post‐discharge: Significant improvement in knowledge medication for both groups (no differences between groups) |
— |
Place of discharge
Discharge planning made little difference to the place of discharge (low certainty), seven studies reported on place of discharge for participants with a medical diagnosis (Goldman 2014; Kennedy 1987; Legrain 2011; Lindpaintner 2013; Moher 1992; Naughton 1994; Sulch 2000), and two studies on place of discharge for participants who were in hospital for a surgical procedure (Evans 1993; Hendriksen 1990) (Analysis 7.1; Analysis 7.2).
7.1. Analysis.
Comparison 7: Effect of discharge planning on place of discharge, Outcome 1: Discharge destination for people with a medical condition
| Discharge destination for people with a medical condition | ||
| Study | Place of discharge | Notes |
| Goldman 2014 | Discharged to a residential care setting: T = 19/347 (5.5%), C = 9/352 (2.6%) Difference 2.9%; 95% CI − 0.04% to 6% |
— |
| Kennedy 1987 | At 2 weeks: 87% no change in placement from time of discharge to 2‐week follow‐up time (both groups) At 4 weeks: majority no change (both groups) | No data shown |
| Legrain 2011 | Discharged home or to a nursing home: T = 183/300 C = 191/339 |
— |
| Lindpaintner 2013 | Discharged home T = 25/30 (83%), C = 30/30 (100%) Difference 17%, 95% CI 2 to 34% |
— |
| Moher 1992 | Discharged home: T = 111/136 (82%), C = 104/131 (79%) Difference 2.2%; 95% CI − 7.3% to 11.7% | — |
| Naughton 1994 | Discharged to nursing home: T = 3/51 (5.9%) C = 2/60 (3.3%) Difference 2.5%; 95% CI − 5.3% to 10.4% | — |
| Sulch 2000 | Discharged home:
T = 56/76 (74%), C = 54/76 (71%) Discharged to an institution: T = 10/76 (13%), C = 16/76 (21%) OR 1.5; 95% CI 0.5 to 2.8 |
— |
7.2. Analysis.
Comparison 7: Effect of discharge planning on place of discharge, Outcome 2: Discharge destination, studies recruiting people with a medical or surgical condition
| Discharge destination, studies recruiting people with a medical or surgical condition | ||
| Study | Place of discharge | Notes |
| Evans 1993 | Discharged to home:
T = 330/417 (79%), C = 305/418 (73%)
P = 0.04 difference 6%; 95% CI 0.39% to 12% Home at 9 months: T = 259/417 (62%), C = 225/418 (54%) P = 0.01 difference 8.3%; 95% CI 1.6% to 15% |
— |
| Hendriksen 1990 | Discharged to nursing home:
T = 0/135 (0%), C = 3/138 (2%)
Difference − 2%; 95% CI − 4.6% to 0.26% At 6 months: admitted to another institution T = 3/135 (2%), C = 14/138 (10%) Difference ‐8%; 95% CI − 13.5% to − 2.3% |
— |
Discussion
Summary of main results
This review assessed the effectiveness of discharge planning in hospital. Thirty‐three randomised trials met the pre‐specified criteria for inclusion. We combined data from trials recruiting older participants with a medical condition and found that discharge planning probably results in a small reduction in hospital length of stay (just under a day; moderate‐certainty evidence) and probably slightly reduces the risk of unscheduled readmissions to hospital (moderate‐certainty evidence) at an average of three months follow‐up. Discharge planning may lead to increased satisfaction for patients and healthcare professionals (low‐certainty evidence, eight trials). It is uncertain whether there is any difference in the cost of care when discharge planning is implemented due to different methods used to cost resources and the year range of the trials that reported data on resource use and cost, ranging from 1994 to 2019 (very lo‐ certainty evidence).
Overall completeness and applicability of evidence
A key issue in interpreting the evidence is variation in how discharge planning was implemented, and the time span of the included studies that ranged from 1990 (Hendriksen 1990) to 2019 (Lisby 2019). The majority of the interventions included a patient education component within the discharge planning process, twenty‐four studies reported active hospital and community liaison to aid timely discharge and an effective transition from hospital to home or another discharge destination. Two of the trials reported using an assessment tool to find cases eligible for discharge planning (Evans 1993; Parfrey 1994). Monitoring of post‐discharge arrangements was mainly done by telephone. The evidence was mixed for the discharge plans that focused on a review and reconciliation of medicines, three reported improvements with medication use between groups (Bolas 2004; Eggink 2010; Shaw 2000), and three trials did not (Farris 2014; Kripalani 2012; Nazareth 2001). The interpretation of these data is limited by the number of different ways that medicine problems were measured.
Local health system factors may impact on how discharge planning is delivered and the configuration of services for the control group. Thirteen of the trials included in this review were based in the USA, five in the UK, three in Canada, one in France, two in Denmark, and one trial each in Australia, Brazil, Slovenia, Sweden, Switzerland, Taiwan, the Netherlands, the Philippines, and Vietnam. In each country the orientation of primary care services differs, which may affect communication between services. The timing of discharge planning during a hospital admission varied across studies, the earlier it is implemented the more time there is for post‐discharge services to be organised. The patient population may also impact on outcome, for example, 99 patients recruited to the trial by Weinberger and colleagues were experiencing major complications from their chronic disease and this, combined with an intervention also designed to increase the intensity of primary care services, may explain the observed increase in re‐admission days for those receiving the intervention. Three trials recruited an ethnically diverse low income and under served population (Goldman 2014; Jack 2009; Balaban 2008) admitted to a hospital that serves diverse communities.
Quality of the evidence
All studies included in this review were randomised controlled trials, we considered most to have a low risk of bias. There was consistency among trials recruiting patients with a medical condition for the main outcomes of readmission and length of stay, and a moderate level of certainty for these outcomes. A small number of studies reported data on cost to the health service and potential cost savings; the findings from these studies is less certain due to different methods for costing resources and the time span of these studies. Few studies assessed patient satisfaction, and of those that did there is some evidence of increased satisfaction in patients experiencing discharge planning.
Potential biases in the review process
Over time discharge planning has been added to interventions that seek to improve care planning, for example comprehensive geriatric assessment (Ellis 2017) and team based inter‐professional interventions (Borenstein 2016). Determining the role of discharge planning in these more complex interventions and selecting studies to include is reliant on the level of reporting in individual studies (Shepperd 2009), this might result in studies being incorrectly categorised as included or excluded. Conversely, there is also a more restrictive application of discharge planning that focuses on medicine reconciliation to prevent medication errors during the transition from hospital to home or another discharge destination (Care Quality Commission 2020; Aronson 2017). A Cochrane EPOC review (Redmond 2016) that assessed the effectiveness of medication reconciliation interventions for improving transitions of care reported very low‐certainty evidence (20 included studies) for a reduction in medicine discrepancies, this review included three of the studies (Bolas 2004; Eggink 2010; Kripalani 2012) we included in our review of discharge planning.
Agreements and disagreements with other studies or reviews
A systematic review of the effectiveness of nurse‐led discharge planning interventions for older people reported that discharge planning increased length of stay by just under a third of a day, and no reduction in readmissions (Mabire 2016). Parker 2002 reviewed discharge planning interventions that were implemented in a hospital setting, these included comprehensive geriatric assessment, discharge support arrangements and educational interventions, concluding that interventions that provided an educational component reduced hospital readmissions. Leppin 2014 reviewed interventions aimed at reducing early hospital readmissions (< 30 days) for adults discharged home versus any other comparator. Their results indicated that interventions that were more complex, promoted patient self‐care and were conducted less recently were more likely to be effective. The authors speculate that an increased standard of care and changes to discharge planning might explain this finding.
Authors' conclusions
Implications for practice.
This review indicates that a structured discharge plan that is tailored to the individual probably brings about a small reduction in hospital length of stay and unscheduled readmission for older people with a medical condition. Discharge planning at an appropriate time in a hospital admission can facilitate the organisation and timely discharge of a patient from hospital and the organisation of post‐discharge services. Even a small reduction in length of stay can be important in freeing up capacity for subsequent admissions in a system where there is a shortage of acute hospital beds. This is reassuring as a potential unintended consequence is that the different steps of a discharge plan might delay discharge if these are implemented sequentially, for example a lengthy assessment is required to inform the discharge plan.
Implications for research.
Some of the stated policy aims of discharge planning, for instance effective communication between the hospital and community services, were not reflected in the outcomes measured in the trials included in this review. Future well‐conducted studies should continue to collect data on readmissions and hospital length of stay, include a qualitative element to the research to explore factors such as communication and transition between care settings, and promote the application of the results by providing details of the intervention and the context in which it was delivered. Investigators should develop safeguards against contamination of the control group, for example by documenting the adoption of discharge planning by the control group.
Feedback
Cochrane Highly Sensitive Search Strategy,
Summary
The Cochrane Highly Sensitive Search Strategy should BE REFERENCED 'Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309:1286‐91' instead of 'Anonymous. MEDLINE optimally sensitive search strategy (OSS) for SilverPlatter. Workshop on Identifying and Registering Trials. UK Cochrane Centre, 1996'.
Reply
This change has now been made.
Contributors
Mike Clarke
What's new
| Date | Event | Description |
|---|---|---|
| 24 February 2022 | New search has been performed | This is the fifth update. We conducted a new search (April 2021), added four new studies and updated review content. We reviewed the previously included studies, removed Pardessus 2000 (N=60) due to a focus on an occupational therapy post‐discharge home visit; and added 4 new studies to the review. A total of 33 studies (N= 12,864 participants) are included in the review. Sources which had not yielded any unique studies over a number of iterations of the search were searched for this update in March 2020 but were not searched for the rerun in April 2021. We reduced the outcomes to seven, removed the outcome 'complications related to hospital admission' as evidence suggests this outcome is less relevant to discharge planning. We limited the number of medication outcomes to adherence and medication problems, and removed hoarding of medicines and medicine knowledge. We divided the outcomes to main outcomes (hospital length of stay, unscheduled readmission to hospital, patient health status: mortality, functional status, psychological health, satisfaction with care and resource use and cost) and secondary outcomes (discharge destination and problems with medication). We restructured the reporting of the results to reflect the Synthesis Without Meta‐analysis (SWiM) reporting guidance. |
| 14 February 2022 | New citation required but conclusions have not changed | This is the fifth update. |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 12 December 2012 | New search has been performed | New search completed March 2012. Three new studies. |
| 7 December 2012 | New citation required but conclusions have not changed | New Search March 2012. Three new studies. |
| 10 November 2009 | New citation required and conclusions have changed | Authors found 10 new studies, providing evidence about the effect of discharge planning. |
| 23 September 2003 | New search has been performed | Search identified additional trials for inclusion |
Acknowledgements
We would like to acknowledge the contribution of EPOC managing editor Chris Cooper, EPOC information specialist Paul Miller and EPOC Contact Editor Andy Oxman. Jeremy Grimshaw and Darryl Wieland for helpful comments on earlier drafts and Luciana Ballini, Tomas Pantoja, Craig Ramsey, Darryl Weiland and Kirsten Woodend for comments on the previous update; Nia Roberts for conducting previous literature searches; and Julie Parkes, Christopher Phillips, Jacqueline McClaran, Sarah Barras, and Annie McCluskey for contributing to previous versions of this review (Parkes 2000; Shepperd 2010, Shepperd 2013).
We would also like to acknowledge the contribution of the following volunteers who translated studies: Ya‐Ying Wang (Chinese), Si Jia Zhu (Chinese), Sven Ruffing (German), Sophia Shakibi (Persian). We thank Andrea Cradduck‐Bamford (ACB) for helping with data extraction and risk of bias assessment for this update.
Appendices
Appendix 1. Search strategies
MEDLINE (Ovid) (1946 to present, MEDALL segment; searched 31 March 2020; 20 April 2021)
| No. | Search terms | Results |
| 1 | ((postdischarge or discharge) adj1 plan*).ti,ab,kf. | 4152 |
| 2 | patient discharge/ | 31764 |
| 3 | ((postdischarge or discharg*) adj4 (plan* or follow up* or home or service? or program* or intervention? or care)).ti,ab,kf. | 35549 |
| 4 | ((postdischarge or discharg*) adj4 (letter? or communicat* or document* or disposition* or status*)).ti,ab,kf. | 6647 |
| 5 | (transition* adj5 (care* or intervention* or home or follow‐up)).ti,ab,kf. | 13169 |
| 6 | (comprehensive adj2 intervention?).ti,ab,kf. | 1992 |
| 7 | or/2‐6 | 75012 |
| 8 | *"continuity of patient care"/ | 10743 |
| 9 | *"length of stay"/ | 12833 |
| 10 | patient readmission/ | 18926 |
| 11 | (readmission* or readmit* or re‐admission* or re‐admit*).ti,ab,kf. | 37484 |
| 12 | (rehospitali* or re‐hospitali*).ti,ab,kf. | 8316 |
| 13 | length of stay.ti,ab,kf. | 62824 |
| 14 | length of hospital stay.ti,ab,kf. | 24696 |
| 15 | ((hospital or hospitali* or bed) adj2 days).ti,ab,kf. | 18312 |
| 16 | hospitali*.ti,ab,kf. | 272473 |
| 17 | or/8‐16 | 385440 |
| 18 | exp randomized controlled trial/ | 528340 |
| 19 | controlled clinical trial.pt. | 94122 |
| 20 | randomi#ed.ti,ab. | 667283 |
| 21 | placebo.ab. | 217179 |
| 22 | randomly.ti,ab. | 356441 |
| 23 | Clinical Trials as topic.sh. | 195530 |
| 24 | trial.ti. | 238373 |
| 25 | or/18‐24 | 1409204 |
| 26 | exp animals/ not humans/ | 4814124 |
| 27 | 25 not 26 | 1299898 |
| 28 | 1 or (7 and 17) | 27955 |
| 29 | 27 and 28 | 2809 |
Embase (Ovid) (1974 to present; searched 31 March 2020; 20 April 2021)
| No. | Search terms | Results |
| 1 | ((postdischarge or discharge) adj1 plan*).ti,ab,kw. | 6137 |
| 2 | *hospital discharge/ | 13468 |
| 3 | ((postdischarge or discharg*) adj4 (plan* or follow up* or home or service? or program* or intervention? or care)).ti,ab,kw. | 68353 |
| 4 | ((postdischarge or discharg*) adj4 (letter? or communicat* or document* or disposition* or status*)).ti,ab,kw. | 12232 |
| 5 | (transition* adj5 (care* or intervention* or home or follow‐up)).ti,ab,kw. | 21537 |
| 6 | (comprehensive adj2 intervention?).ti,ab,kw. | 2736 |
| 7 | or/2‐6 | 104976 |
| 8 | *patient care/ | 69877 |
| 9 | *hospital readmission/ | 14955 |
| 10 | *"length of stay"/ | 12380 |
| 11 | (readmission* or readmit* or re‐admission* or re‐admit*).ti,ab,kw. | 70809 |
| 12 | (rehospitali* or re‐hospitali*).ti,ab,kw. | 15290 |
| 13 | length of stay.ti,ab,kw. | 118743 |
| 14 | length of hospital stay.ti,ab,kw. | 39991 |
| 15 | ((hospital or hospitali* or bed) adj2 days).ti,ab,kw. | 32727 |
| 16 | hospitali*.ti,ab,kw. | 444818 |
| 17 | or/8‐16 | 685394 |
| 18 | 1 or (7 and 17) | 38793 |
| 19 | random*.ti,ab. | 1674609 |
| 20 | factorial*.ti,ab. | 41277 |
| 21 | (crossover* or cross over*).ti,ab. | 113267 |
| 22 | ((doubl* or singl*) adj blind*).ti,ab. | 247168 |
| 23 | (assign* or allocat* or volunteer* or placebo*).ti,ab. | 1111335 |
| 24 | crossover procedure/ | 66940 |
| 25 | single blind procedure/ | 42811 |
| 26 | randomized controlled trial/ | 668808 |
| 27 | double blind procedure/ | 185158 |
| 28 | or/19‐27 | 2512415 |
| 29 | exp animal/ not human/ | 4950893 |
| 30 | 28 not 29 | 2264766 |
| 31 | 18 and 30 | 4717 |
Cochrane Central Register of Controlled Trials (CENTRAL) via Cochrane Library (Wiley) (searched 31 March 2020; 20 April 2021)
| No. | Search terms | Results |
| #1 | ((postdischarge or discharge) near/1 plan*):ti,ab,kw | 494 |
| #2 | [mh "patient discharge"] | 1541 |
| #3 | ((postdischarge or discharg*) near/4 (plan* or (follow next up*) or home or service? or program* or intervention? or care)):ti,ab,kw | 8289 |
| #4 | ((postdischarge or discharg*) near/4 (letter? or communicat* or document* or disposition* or status*)):ti,ab,kw | 961 |
| #5 | (transition* near/5 (care* or intervention* or home or (follow next up))):ti,ab,kw | 1763 |
| #6 | (comprehensive near/2 intervention?):ti,ab,kw | 892 |
| #7 | {or #2‐#6} | 11784 |
| #8 | [mh ^"continuity of patient care"] | 617 |
| #9 | [mh "length of stay"] | 7368 |
| #10 | [mh "patient readmission"] | 1084 |
| #11 | (readmission* or readmit* or (re‐admission*) or (re‐admit*)):ti,ab,kw | 7778 |
| #12 | (rehospitali* or re‐hospitali*):ti,ab,kw | 2528 |
| #13 | length of stay:ti,ab,kw | 20096 |
| #14 | length of hospital stay:ti,ab,kw | 6696 |
| #15 | ((hospital or hospitali* or bed) near/2 days):ti,ab,kw | 4912 |
| #16 | hospitali*:ti,ab,kw | 55659 |
| #17 | {or #8‐#16} | 77771 |
| #18 | #1 or (#7 and #17) | 661 |
CINAHL (EBSCO) (1982 to present; searched 31 March 2020)
| No. | Search terms | Results |
| S1 | TI ((postdischarge or discharge) N1 plan*) | 1,209 |
| S2 | (MH "Discharge Planning+") | 5,442 |
| S3 | S1 OR S2 | 5,788 |
| S4 | (MH "Patient Discharge") OR (MH "Early Patient Discharge") OR (MH "Patient Discharge Education") OR (MH "Transfer, Discharge") | 27,514 |
| S5 | ((postdischarge or discharg*) N4 (plan* or follow up* or home or service? or program* or intervention? or care)) | 23,834 |
| S6 | ((postdischarge or discharg*) N4 (letter? or communicat* or document* or disposition* or status*)) | 3,739 |
| S7 | (transition* N5 (care* or intervention* or home or follow‐up)) | 11,700 |
| S8 | (comprehensive N2 intervention?) | 1,683 |
| S9 | S4 OR S5 OR S6 OR S7 OR S8 | 57,243 |
| S10 | (MM "Continuity of Patient Care") | 7,518 |
| S11 | (MM "Length of Stay") | 6,815 |
| S12 | (MH "Readmission") | 13,964 |
| S13 | (readmission* or readmit* or re‐admission* or re‐admit*) | 22,217 |
| S14 | (rehospitali* or re‐hospitali*) | 3,166 |
| S15 | length of stay | 60,567 |
| S16 | length of hospital stay | 21,591 |
| S17 | hospitali* | 111,635 |
| S18 | S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 | 177,893 |
| S19 | S9 AND S18 | 19,528 |
| S20 | S3 OR S19 | 23,343 |
| S21 | PT randomized controlled trial | 129,604 |
| S22 | PT clinical trial | 108,702 |
| S23 | TI ( randomis* or randomiz* or randomly) OR AB ( randomis* or randomiz* or randomly) | 317,542 |
| S24 | (MH "Clinical Trials+") | 315,031 |
| S25 | (MH "Random Assignment") | 67,358 |
| S26 | S21 OR S22 OR S23 OR S24 OR S25 | 491,516 |
| S27 | S20 AND S26 | 2,102 |
PsycInfo (OvidSP) (1967 to present; searched 31 March 2020)
| No. | Search terms | Results |
| 1 | ((postdischarge or discharge) adj1 plan*).ti,ab. | 1095 |
| 2 | discharge planning/ | 414 |
| 3 | or/1‐2 | 1263 |
| 4 | hospital discharge/ | 2171 |
| 5 | ((postdischarge or discharg*) adj4 (plan* or follow up* or home or service? or program* or intervention? or care)).ti,ab. | 5632 |
| 6 | ((postdischarge or discharg*) adj4 (letter? or communicat* or document* or disposition* or status*)).ti,ab. | 952 |
| 7 | (transition* adj5 (care* or intervention* or home or follow‐up)).ti,ab. | 5810 |
| 8 | (comprehensive adj2 intervention?).ti,ab. | 1096 |
| 9 | or/4‐8 | 14118 |
| 10 | "continuum of care"/ | 1794 |
| 11 | (readmission* or readmit* or re‐admission* or re‐admit*).ti,ab. | 3539 |
| 12 | (rehospitali* or re‐hospitali*).ti,ab. | 1947 |
| 13 | length of stay.ti,ab. | 4976 |
| 14 | length of hospital stay.ti,ab. | 880 |
| 15 | ((hospital or hospitali* or bed) adj2 days).ti,ab. | 1682 |
| 16 | hospitali*.ti,ab. | 45705 |
| 17 | or/10‐16 | 55081 |
| 18 | 3 or (9 and 17) | 3980 |
| 19 | exp clinical trial/ | 12031 |
| 20 | random*.ti,ab. | 196974 |
| 21 | ((clinical or control*) adj3 trial*).ti,ab. | 73029 |
| 22 | ((singl* or doubl* or trebl* or tripl*) adj5 (blind* or mask*)).ti,ab. | 26165 |
| 23 | (volunteer* or control group or controls).ti,ab. | 244292 |
| 24 | placebo/ or placebo*.ti,ab. | 40038 |
| 25 | or/19‐24 | 453650 |
| 26 | 18 and 25 | 508 |
ClinicalTrials.gov (searched 31 March 2020; 20 April 2021)
| Fields | Search terms |
| Intervention/treatment: | discharge plan* |
WHO ICTRP (searched 20 April 2021)
| Search terms |
| discharge plan* |
Data and analyses
Comparison 1. Effect of discharge planning on hospital length of stay.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Hospital length of stay ‐ older people with a medical condition | 11 | 2113 | Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐1.33, ‐0.12] |
| 1.2 Hospital length of stay ‐ older people following surgery | 2 | 184 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐1.23, 1.11] |
| 1.3 Hospital length of stay ‐ studies recruiting people with a mix of conditions | 3 | Other data | No numeric data |
Comparison 2. Effect of discharge planning on unscheduled readmission rates.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Average follow‐up, 3 months from discharge for the majority of studies | 17 | 5126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.81, 0.97] |
| 2.1.1 Unscheduled readmission for participants with a medical condition | 17 | 5126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.81, 0.97] |
| 2.2 Hospital readmission rates at various follow‐up times | 18 | Other data | No numeric data | |
| 2.2.1 Participants with a medical condition | 14 | Other data | No numeric data | |
| 2.2.2 Participants with medical or surgical condition | 1 | Other data | No numeric data | |
| 2.2.3 Participants recruited following surgery | 2 | Other data | No numeric data | |
| 2.2.4 Participants with a mental health diagnosis | 2 | Other data | No numeric data |
2.1. Analysis.

Comparison 2: Effect of discharge planning on unscheduled readmission rates, Outcome 1: Average follow‐up, 3 months from discharge for the majority of studies
Comparison 3. Effect of discharge planning on health status.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Mortality at 3 to 9 months | 8 | 2721 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.85, 1.29] |
| 3.1.1 Older people with a medical condition | 8 | 2721 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.85, 1.29] |
| 3.2 Mortality for trials recruiting participants with a medical condition and those recovering from surgery | 1 | Other data | No numeric data | |
| 3.3 Patient‐reported outcomes: a medical condition | 15 | Other data | No numeric data | |
| 3.3.1 Patients with a medical condition | 12 | Other data | No numeric data | |
| 3.3.2 Patient report outcomes following surgery | 2 | Other data | No numeric data | |
| 3.3.3 Patients with a medical or surgical condition | 1 | Other data | No numeric data | |
| 3.3.4 Patients with a mental health diagnosis | 1 | Other data | No numeric data |
Comparison 4. Effect of discharge planning on satisfaction with care process.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Satisfaction | 8 | Other data | No numeric data | |
| 4.1.1 Patient and care givers' satisfaction | 7 | Other data | No numeric data | |
| 4.1.2 Professional's satisfaction | 2 | Other data | No numeric data |
Comparison 5. Effect of discharge planning on hospital resource use and cost.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Hospital cost | 6 | Other data | No numeric data | |
| 5.1.1 Patients with a medical condition | 6 | Other data | No numeric data | |
| 5.1.2 Patients with a surgical condition | 1 | Other data | No numeric data | |
| 5.2 Primary and community care resource use and cost | 6 | Other data | No numeric data |
Comparison 6. Effect of discharge planning on medication use.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Problems with medication after discharge from hospital | 6 | Other data | No numeric data | |
| 6.2 Adherence to medicines | 4 | Other data | No numeric data | |
| 6.3 Knowledge about medicines | 3 | Other data | No numeric data |
Comparison 7. Effect of discharge planning on place of discharge.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Discharge destination for people with a medical condition | 7 | Other data | No numeric data | |
| 7.2 Discharge destination, studies recruiting people with a medical or surgical condition | 2 | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Balaban 2008.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between June 2006 and January 2007 |
|
| Participants | A culturally and linguistically diverse group of patients who were admitted to hospital as an emergency, and had to have a 'medical home' defined as having an established primary care provider to be discharged to; patients were excluded if previously enrolled in the study, discharged to another institution or residing in long‐term care facility. Number of patients recruited: T = 47, C = 49 Number with diabetes: T = 12/47, C = 18/49 Number with heart failure: T = 5/47, C = 5/49 Number with COPD: T = 6/47, C = 6/49 Number with depression: T = 23/47, C = 19/49 Number of patients recruited: T = 47, C = 49 Mean age: T = 58 years, C = 54 years Sex (female): T = 27/47 (57.4%), C = 30/49 (61%) Non‐English‐speaking: T = 19/47 (40%), C = 9/49 (18.4%) |
|
| Interventions |
Setting: a safety net 100‐bed community teaching hospital affiliated with Harvard Medical School, USA Pre‐admission assessment: no Case finding on admission: enrolled at admission Inpatient assessment and preparation of a discharge plan based on individual patient needs: a comprehensive Patient Discharge Form was provided to patients in one of 3 languages (English, Spanish and Portuguese). The form sought to identify communication problems that occur during the transition of care, including patients' lack of knowledge about their condition and any gaps in outpatient follow‐up care or follow‐up of test results. Implementation of the discharge plan: the Discharge Form was electronically transferred to the RN at the patient's primary care facility, a primary care RN contacted the patient and reviewed the Discharge Form and the medication included in the discharge‐transfer plan Monitoring phase: by primary care RN who telephoned the patient to assess their medical status, review the Patient Discharge Form, assess patient concerns and confirm scheduled follow‐up appointments. Immediate interventions were arranged as needed, and the discharge form and telephone notes were forwarded electronically to the primary care provider who reviewed the form. Control: discharged according to existing hospital practice, which consisted of receiving discharge instructions handwritten in English. Communication between the discharge physician and primary care physician was done on an as‐needed basis. |
|
| Outcomes | Hospital length of stay and readmission rates Follow‐up at 21 and 31 days |
|
| Notes |
Funding: grant from the CRICO/Risk Management Foundation Conflicts of interest: none reported Ethical approval: Institutional Review Board 24/120 patients were excluded after randomisation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Baseline outcome data | Low risk | Comment: not applicable as main outcomes were length of stay and readmission rates |
| Baseline characteristics similar | Unclear risk | Comment: the groups were similar for the majority of baseline characteristics. Baseline characteristics were collected and reported; groups were similar for age, sex, length of hospital stay and chronic medical conditions, however those allocated to the intervention group were more likely to be non‐English speakers and discharged during the weekend (Table 1; p.1230, 2nd column) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Main outcome measure was readmission rates |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Follow‐up data for > 80% |
| Study adequately protected against contamination | Unclear risk | Comment: Patients recruited from the same floor were allocated to the groups; intervention was delivered by the same personnel delivering care to those allocated to the comparison group (p.1229, top 1st column); there was no evidence that the intervention discharge form was used for the control group |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not able to judge from available information |
| Other bias | Low risk | Comment: Not reported |
Bolas 2004.
| Study characteristics | ||
| Methods | Parallel randomised trial Not reported when study was conducted |
|
| Participants | Patients recruited within 48 hours of an emergency or unplanned admission to the medical admissions unit, aged ≥ 55 years and taking 3 regular drugs or more. Patients were excluded if transferred to another hospital, admitted or transferred to a nursing home, if patient or caregiver was unable to communicate with pharmacist, had mental illness or alcohol‐related admission, or if home visit or follow‐up was declined on admission. Number of patients recruited: T = 119, C = 124 Mean age: T = 73 years, C = 75 years Sex (female): T = 41/119 (34%), C = 42/124 (34%) Living alone: T = 27/119, C = 34/124 |
|
| Interventions |
Setting: Antrim Hospital, a 426‐bed district general hospital in Northern Ireland Pre‐admission assessment: no Case finding on admission: not described Inpatient assessment and preparation of a discharge plan based on individual patient needs: use of a comprehensive medication history service, provision of an intensive clinical pharmacy service including management of patients' own drugs brought to hospital, personalised medicines record and patient counselling to explain changes at discharge. Implementation of the discharge plan: discharge letter outlining complete drug history on admission and explanation of changes to medication during hospital and variances to discharge prescription. This was faxed to GP and community pharmacist. Personalised medicine card, discharge counselling, labelling of dispensed medications under the same headings for follow‐up Monitoring: medicines helpline Control: standard clinical pharmacy service |
|
| Outcomes | Patient satisfaction, knowledge of medicines, hoarding of medicines Readmissions and length of stay data not reported |
|
| Notes |
Funding: Primary Care Development Fund, Northern Ireland Conflicts of interest: not reported Ethical approval: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Computer‐generated random number |
| Allocation concealment (selection bias) | Unclear risk | Comment: Allocation concealment was not described |
| Baseline outcome data | Unclear risk | Comment: Outcome data not reported |
| Baseline characteristics similar | Low risk | Comment: Baseline data reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Low risk for readmission data |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Follow‐up of patients: 67% (162/243) Low response rate in survey of GPs (55% response rate) and community pharmacists (56% response rate) |
| Study adequately protected against contamination | Unclear risk | Comment: Participants were recruited from the same medical unit or emergency department; unclear how the intervention was delivered |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not able to judge from available information |
| Other bias | Low risk | Comment: Not reported |
Bonetti 2018.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between February and December 2015 |
|
| Participants | Patients aged >=18 years admitted to a specialised cardiology ward due to stable angina, acute coronary syndrome, congestive heart failure, valvular disease, arrhythmias, or hypertension Number of patients randomised: 133 (T: 66, C: 67); Analysed: 102 (primary endpoint; I: 51, C: 51) Mean age: T: 65 years (SD 10), C: 65 years (SD 13) Sex (female): T = 16/51 (31%), C = 19/53 (36%) Other relevant characteristics: On average participants had 4 co‐morbidities, took 7.5 medications at discharge and were in hospital for 11 days |
|
| Interventions |
Setting: Tertiary hospital, Curitiba, Brazil Pre‐admission assessment: no Case finding on admission: cardiovascular pharmacy residents assessed patients eligibility according to the eligibility criteria Inpatient assessment and preparation of a discharge plan based on individual patient needs: two cardiovascular pharmacists provided individual counselling sessions (number not specified) to the patient and their carer, if applicable. The sessions included a medication needs assessment, as well as an educational component covering indications and possible adverse drug events, among other topics. Implementation of the discharge plan: patients were given a personalised leaflet summarising the information covered by the sessions. Monitoring phase: patients were contacted by telephone to reinforce the previous counselling session (3 and 15 days post‐discharge) Control: usual care, provided by pharmacists and other healthcare providers |
|
| Outcomes | Main outcomes: emergency department visits (related to heart disease, not related to heart disease), total hospital readmission, hospital readmission (related to heart disease, not related to heart disease), mortality Other outcomes: drug taking procedures, beliefs about medicine, medication adherence, number of medication problems Follow‐up at 30 days |
|
| Notes |
Funding: not reported Conflicts of interest: no potential conflict of interest was reported. Ethical approval: “This trial was in accordance with the ethical standards of the institution’s committee.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “random number list (...) using Microsoft Office Excel 2010” (Methods) |
| Allocation concealment (selection bias) | Low risk | Quote: “generated by a third person” (Methods) |
| Baseline outcome data | Low risk | Comments: Groups similar for days of hospitalisation and number of comorbidities. Other main outcomes referred to ED visits, readmission, and mortality (Table 1) |
| Baseline characteristics similar | Low risk | Comment: Baseline characteristics presented and similar between groups (Table 1) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: how data for the main outcomes were collected isn't clear (methods) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Attrition rate high albeit similar between groups (IG: 23%, CG: 21%). Unclear why participants were lost to ambulatory follow‐up (Fig.1) |
| Study adequately protected against contamination | High risk | Quote: “There were five trained pharmacists in this setting, including one of the residents who provided the intervention.” (Methods) |
| Selective reporting (reporting bias) | Unclear risk | Comment: We identified two publications, which refer to different outcomes, neither lists all outcomes collected for the study |
| Other bias | Low risk | Comment: No other apparent risk of bias |
Cajanding 2017.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between August 2013 and August 2014 |
|
| Participants | Patients aged >18 years, with AMI diagnosed according to established guidelines, admitted to the study hospital for AMI treatment. Patients were excluded if they were admitted for other co‐morbidities, were medically unstable, and were unable to read or write English. Number of patients recruited: T: 107, C: 92; Analysed: T: 75; C: 68 Age: participants' age ranged between 31 and 74 years; most were aged between 51 and 60 years old. Sex (female): T = 27/75 (36%), C = 26/68 (38%) No previous myocardial infarction: T = 59/75 (79%), C = 55/68 (81%) |
|
| Interventions |
Setting: cardiovascular‐coronary care unit of a comprehensive tertiary referral hospital in Manila, the Philippines Pre‐admission assessment: no Case finding on admission: all patients admitted for AMI treatment were invited to participate Inpatient assessment and preparation of a discharge plan based on individual patient needs: starting on the 2nd day of hospitalisation, each patient had 3 sessions (30 to 45 minutes) in 3 consecutive days with a cardiovascular nurse practitioner. Sessions addressed risk and protective factors of cardiovascular disease, medication compliance and physical activity, among other topics. During the third session an action plan booklet is completed, with goals and action plans, and establishment of a contract between the nurse and the patient. Patients completed measures of perceived functional status, cardiac self‐efficacy and patient satisfaction. Implementation of the discharge plan: patients were given an action plan booklet, with the goals and action plans previously developed with the nurse. Monitoring: unclear; authors state that patients were asked to bring the booklet with them for their follow‐up visits, but not clearly described. Control: traditional care, based on the Philippine Heart Association clinical practice guidelines. Included medical and pharmacological therapy, as well as preventable risk factor modifications strategies for AMI, as prescribed by the patient's primary cardiologist. Participants allocated to the intervention group also received traditional care. |
|
| Outcomes |
Self‐reported: perceived functional status, self‐efficacy, patient satisfaction Records review: unexpected hospital visits (including readmissions, emergency department visits, outpatient department visits, and general practitioner visits) Follow‐up at 30 days |
|
| Notes |
Funding: no funding to disclose Conflicts of interest: none reported Ethical approval: granted by graduate nursing education department and institutional ethics committee Notes: Authors developed a structured handbook with FAQs to guide programme implementation and enhance fidelity |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “computerized random‐number generator” (p.69, top 2nd column) |
| Allocation concealment (selection bias) | Unclear risk | Comment: not enough information provided to make a judgement. |
| Baseline outcome data | Low risk | Comment: baseline outcome data presented for functional status, self‐efficacy and patient satisfaction, and balanced between groups (Table 3) |
| Baseline characteristics similar | Low risk | Comment: baseline characteristics presented and balanced between groups (Table 2) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: “Blinding was strictly observed for the data collection phase in this study. Except for the interventionists, the rest of the investigators were kept blind to the group assignment of the participants. The investigators who obtained the baseline and the outcome measures were not informed of the participant’s group assignments." (p.69, mid 1st column) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: high attrition rate (IG: 30%, CG: 26%), most participants either refused to answer follow‐up questionnaire or were lost to follow‐up |
| Study adequately protected against contamination | Unclear risk | Quote: “Ward nurses were not informed of any patient’s allocation, and efforts were made to keep the conduct of the intervention private and concealed to the regular care staff.” (p.69, mid 2st column) |
| Selective reporting (reporting bias) | Low risk | Comment: No evidence of selective reporting |
| Other bias | Unclear risk | Comment: Not reported |
Eggink 2010.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between May 2007 and July 2008 |
|
| Participants | Patients aged ≥ 18 years, with heart failure who were prescribed ≥ 5 medicines at discharge; patients were excluded if living in a nursing home or unable to provide informed consent. Number of patients recruited: T = 41, C = 44 Mean age (SD): T = 74 (12), C = 72 (10) Sex (female): T = 14/41 (41%), C = 11/44 (25%) |
|
| Interventions |
Setting: Department of Cardiology in a teaching hospital in Tilburg, the Netherlands Pre‐admission assessment: no Case finding on admission: not described Inpatient assessment and preparation of a discharge plan based on individual patient needs: the clinical pharmacist identified potential prescription errors in the discharge medication, developed a discharge medication list and discussed with the cardiologist. Implementation of the discharge plan: patients received verbal and written information about side effects and changes in their hospital drug therapy from a clinical pharmacist at discharge. A discharge medication list was faxed to the community pharmacy and given as written information to the patient; this contained information on dose adjustments and discontinued medications. Monitoring: not described Control: regular care, verbal and written information about their drug therapy from a nurse at hospital discharge, the prescription was made by the physician and given to the patient to give to the GP |
|
| Outcomes | Adherence to medication, prescribing errors (an error in the process of prescribing) and discrepancies (a restart of a discontinued medication, discontinuation of prescribed discharge medication, use of higher or lower dose, more or less frequent use than prescribed and incorrect time of taking medication) | |
| Notes |
Funding: no funding was received for this study Conflicts of interest: none reported Ethical approval: mMedical ethics committee |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Random number table |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Baseline outcome data | Low risk | Comment: Baseline outcome data provided for number of medications and patient control over medications at discharge was similar between groups (Table 3) |
| Baseline characteristics similar | Low risk | Comment: Majority of characteristics similar at baseline (Table 3) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Low risk for count of prescribing errors, unclear risk for adherence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Loss to follow‐up = 2/89 |
| Study adequately protected against contamination | Low risk | Comment: The clinical pharmacist who delivered the intervention had no contact with participants allocated to the comparison group |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not able to judge from available information |
| Other bias | Unclear risk | Comment: Not reported |
Evans 1993.
| Study characteristics | ||
| Methods | Parallel randomised trial Not reported when study was conducted |
|
| Participants | Patients aged ≥ 70 years and admitted with a medical condition, neurological condition, or recovering from surgery, were screened for risk factors that would prolong their hospital length of stay Number of patients recruited: T = 417, C = 418 Mean age: T = 66.6 years, C = 67.9 years |
|
| Interventions |
Setting: Veterans Affairs Hospital, Seattle, USA Pre‐admission assessment: no Case finding on admission: patients screened for risk factors that may prolong length of stay, increase risk of readmission, or discharge to a nursing home Inpatient assessment and preparation of a discharge plan based on individual patient needs: during discharge planning. information on support systems, living situation, finances and areas of need were obtained from the medical notes; interviews with the patient and family, and consulting with the physician and nurse Implementation of the discharge plan: discharge planning initiated on day 3 of hospital admission, and these patients were referred to a social worker. Plans were implemented with measurable goals using goal attainment scaling. Monitoring: not reported Control: received discharge planning only if referred by medical staff and usually on the 9th day of hospital admission, or not at all |
|
| Outcomes | Hospital length of stay, readmission to hospital, discharge destination, health status Follow‐up at 3 months |
|
| Notes |
Funding: Department of Veterans Affairs Health Service Research & Development Program Conflicts of interest: not reported Ethical approval: not reported Also validated an instrument to assess high‐risk patients Intervention implemented on day 3 of hospital admission |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Baseline outcome data | Low risk | Comment: Baseline outcome data presented for health status, hospital admissions in the past 3 months and place of living, and similar between groups (Table 2) |
| Baseline characteristics similar | Low risk | Comment: Baseline data reported and similar between groups (Table 2) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Yes, for objective measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All patients randomised accounted for at follow‐up |
| Study adequately protected against contamination | Unclear risk | Comment: Not reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not able to judge from available information |
| Other bias | Low risk | Comment: Not reported |
Farris 2014.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between March 2008 and October 2012 |
|
| Participants | Patients aged ≥ 18 years, English‐ or Spanish‐ speaking, admitted with diagnosis of hypertension, hyperlipidaemia, HF, coronary artery disease, MI, stroke, TIA, asthma, COPD or receiving oral anticoagulation, with life expectancy of ≥ 6 months and without cognitive impairment, dementia or severe psychiatric diagnosis Number of patients recruited: enhanced T = 314, minimum T = 315, C = 316 Mean age (SD): 61.0 (12.2) |
|
| Interventions |
Setting: Academic health centre, Iowa, USA Pre‐admission assessment: no Case finding on admission: electronic medical records screened for eligibility, followed by patient screening Inpatient assessment and preparation of a discharge plan based on individual patient needs: patients in Minimum and Enhanced Intervention received admission medication reconciliation and pharmacist visits every 2 to 3 days during inpatient stay for education Implementation of the discharge plan: patients allocated to the Minimum and Enhanced Intervention received counselling and a discharge medication list; counselling was tailored to the individual and focused on goals of therapy, medication administration, barriers to adherence that included cost and patient concerns. PCP and community pharmacist of patients in Enhanced Intervention received a copy of the discharge plan (6 to 24 hours postdischarge) with a medication list and patient‐specific concerns. Monitoring: patients in the Enhanced Intervention group received a call 3 to 5 days postdischarge Control: medication reconciliation at admission as per hospital policy, nurse discharge counselling and discharge medication list. The discharge summary was transcribed and received in the mail by the PCP several days or weeks after discharge. |
|
| Outcomes | Medication appropriateness, adverse events, preventable adverse events, composite variable of combined hospital readmission, emergency department visit or unscheduled office visit. Follow‐up at 30 and 90 d postdischarge | |
| Notes |
Funding: National Heart, Lung, and Blood Institute Conflicts of interest: 2/10 authors, including the lead author reported consultancy work with public higher education institutions; the lead author reported honoraria and travel expenses from a pharmaceutical company for presentation and article about pharmacists in care transitions. Ethical approval: Institutional Review Board Notes: Fidelity assessment conducted to assess which intervention components were delivered |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Statistician‐generated blinded randomisation scheme, sequentially numbered envelopes |
| Allocation concealment (selection bias) | Low risk | Comment: Unit of allocation by patient, with sealed opaque envelope |
| Baseline outcome data | Low risk | Comment: Baseline outcome data reported for average number of prescriptions, self‐reported medication adherence and medication management; control group less likely to forget medication but not related with main outcome (Table 1) |
| Baseline characteristics similar | Low risk | Comment: Baseline characteristics reported, similar between groups (Table 1) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: Pharmacists unaware of patients allocation to Minimum Intervention or Enhanced Intervention until discharge; status of RAs who assessed baseline and follow‐up unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 9 patients lost to follow‐up (3 per group: Enhanced Intervention = 311/314; Minimum Intervention = 312/315; Control = 313/316) |
| Study adequately protected against contamination | Low risk | Comment: Intervention was delivered by the pharmacy case managers, who did not have contact with participants allocated to the comparison group. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Some of the secondary outcomes were analysed in aggregate; however, they were also reported separately and it was possible to extract sufficient information |
| Other bias | Low risk | Comment: Not reported |
Gillespie 2009.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between September 2005 and June 2007 |
|
| Participants | Patients aged ≥ 80 years, admitted to 2 internal medicine wards; excluded if admitted previously to the study wards during the study period or had scheduled admissions Number of patients recruited: T = 182, C = 186 Mean age (SD): T = 86.6 (4.2), C = 87.1 (14.1) Sex (female): T = 105 (57.7%), C = 111 (59.7%) |
|
| Interventions |
Setting: teaching hospital, Uppsala, Sweden Pre‐admission assessment: no Case finding on admission: no Inpatient assessment and preparation of a discharge plan based on individual patient needs: study pharmacists compiled a comprehensive list of current medications, after which they reviewed the drugs. Advice on drug selection, dosages, and monitoring needs was given to the patient's physician, who was responsible for the final decision. Patients were educated and monitored throughout the admission process Implementation of discharge plan: PCP contacted and given discharge medications, which included rationale for changes and monitoring needs for newly commenced drugs. All information was approved by ward physicians Monitoring: follow‐up telephone call to patients 2 months after discharge Control: standard care without pharmacists' involvement in the healthcare team at the ward level |
|
| Outcomes | Frequency of hospital visits 12 months after (last included patient) discharge from hospital; number of readmissions, ED visits, and costs | |
| Notes |
Funding: Uppsala County Council, University Hospital of Uppsala, Uppsala University, Apoteket AB, and Swedish Society of Pharmaceutical
Sciences Conflicts of interest: none reported Ethical approval: regional ethics committee |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Randomisation was performed in blocks of 20 (each block contained 10 intervention and 10 control allocations) |
| Allocation concealment (selection bias) | Low risk | Comment: Block randomisation with a closed‐envelope technique. The randomisation process was performed by the clinical trials group at the Hospital Pharmacy. |
| Baseline outcome data | Low risk | Comment: Outcome events occured after the intervention and discharge from hospital |
| Baseline characteristics similar | Low risk | Comment: Baseline characteristics reported and similar between groups (Table 1) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Objective measures of outcome using routine data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: T: 13 died before discharge and 4 withdrew; C: 14 died and 1 withdrew (< 8%) |
| Study adequately protected against contamination | Low risk | Comment: The intervention was delivered by clinical pharmacists who did not have contact with participants allocated to the comparison group |
| Selective reporting (reporting bias) | Low risk | Comment: Main outcome is the same as reported for the trial registry (https://clinicaltrials.gov/show/NCT00661310) |
| Other bias | Low risk | Comment: Not reported. |
Goldman 2014.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between July 2010 and February 2013 |
|
| Participants | Patients aged ≥ 60 years (later lowered to 55 to improve recruitment), admitted unexpectedly to the internal or family medicine, cardiology, or neurology departments; English‐, Spanish‐ or Mandarin‐speaking, likely to be discharged home and able to consent Number of patients recruited: T = 347, C = 352 Mean age (SD): T = 66.5 years (9.0), C = 66.0 years (9.0) Sex (female): T = 159/347 (46%), C = 145/352 (41%) |
|
| Interventions |
Setting: safety‐net hospital, San Francisco, USA Pre‐admission assessment: no Case finding on admission: electronic medical records screened for eligibility, followed by meeting with attending physician Inpatient assessment and preparation of a discharge plan based on individual patient needs: RN provided disease‐specific patient education either in the patient's preferred language or via a trained interpreter; motivational interviewing and coaching for engagement; written materials provided Implementation of discharge plan: from admission to discharge, with outreach visit by RN within 24 h of discharge; PCP contacted and given inpatient physicians' contact. Monitoring: NP called patients 1 to 3 and 6 to 10 days after discharge to assess adherence to medication, provide further education if required, help solve barriers to attending follow‐up appointments, among others Control: bedside RN's review of the discharge instructions, received by all patients. If requested by the medical team, the hospital pharmacy provided a 10 ‐day medication supply and a social worker assisted with discharge. The admitting team was responsible for liaising with the patients' PCP |
|
| Outcomes | ED visits or readmissions (30, 90 and 180 days), non‐ED ambulatory care visits, mortality (180 days) | |
| Notes |
Funding: Gordon and Betty Moore Foundation, USA Conflicts of interest: 1/10 authors reported receiving lecture fees from a federal quality improvement programme Ethical approval: not reported Notes: fidelity assessment conducted to measure which intervention components were delivered. Age criterion was changed halfway from ≥ 60 to ≥ 55 years to increase the number of eligible participants. Authors provided supplementary data (readmissions and ED visits were presented as an aggregated outcome, access provided to separate outcomes) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Statistician‐generated randomised tables of treatment assignment in blocks of 50 for each language |
| Allocation concealment (selection bias) | Low risk | Comment: Pairs of envelopes containing the treatment assignment and labelled with the study identification number |
| Baseline outcome data | Low risk | Comment: Emergency department visits and hospitalisations for 6 months prior to baseline reported and similar between groups (Table 1) |
| Baseline characteristics similar | Low risk | Comment: Baseline data reported (Table 1) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Blinded outcome assessment and objective primary outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Follow‐up at 180 d = 90%. All drop‐outs accounted for |
| Study adequately protected against contamination | Unclear risk | Comment: Participants allocated to intervention and comparison groups within the same wards; unclear whether the same study research nurse delivers intervention and comparison groups (p.473) |
| Selective reporting (reporting bias) | Low risk | Comment: Trial registration provides same primary outcomes as reported here |
| Other bias | Low risk | Comment: Not reported. |
Harrison 2002.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between June 1996 and January 1998 |
|
| Participants | Patients admitted with CHF, who lived within the regional home care radius (60 km), were expected to be discharged to home nursing care and were not cognitively impaired Number of patients recruited: T = 92, C = 100 Mean age (SD): T = 75.5 years (10.4), C = 75.7 years (9.7) Sex (female): T = 43/92 (47%), C = 44/100 (44%) |
|
| Interventions |
Setting: large urban teaching hospital, Ottawa, Canada Pre‐admission assessment: no Case finding on admission: patients' notes were flagged as a signal to the primary nurse to follow a checklist for Transitional Care Inpatient assessment and preparation of a discharge plan based on individual patient needs: comprehensive discharge planning, which included hospital and community nurses working together to smooth transition from hospital to home (Transitional Care intervention); a structured evidence based protocol was used for counselling and education for heart failure self‐management (Partners in Care for Congestive Heart Failure). The protocol followed AHCPR guidelines. Home nursing visits ‐ the same number as the control group. Implementation of discharge plan: from admission to discharge, with telephone outreach within 24 hours of discharge Monitoring: not reported Control: received usual care for hospital‐to‐home transfer, which involved completion of a medical history, nursing assessment form and a multidisciplinary plan. Discharge planning meetings took place weekly. A regional home care coordinator consulted with the hospital team as required. Patients received the same number of home nurse visits as the intervention group. |
|
| Outcomes | Health‐related quality of life, symptom distress and functioning. Emergency room visits and readmissions at 12 weeks. | |
| Notes |
Funding: Health Canada, National Health Research and Development Program, Canada Conflicts of interest: not reported Ethical approval: Institutional Ethics Review Board |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Computer‐generated schedule of random numbers |
| Allocation concealment (selection bias) | Low risk | Comment: Random allocation by a research co‐ordinator |
| Baseline outcome data | Low risk | Comment: Baseline outcome data reported and similar for most outcomes between groups (Table 3), slightly higher admission rate to hospital in the previous six months |
| Baseline characteristics similar | Low risk | Comment: Baseline data reported and similar between groups (Table 2) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Low risk for objective measure of readmission |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 157/200 (81%) completed the study |
| Study adequately protected against contamination | Low risk | Comment: The control group did not have access to the intervention |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not able to judge from available information |
| Other bias | Low risk | Comment: Not reported |
Hendriksen 1990.
| Study characteristics | ||
| Methods | Parallel randomised trial Study dates: not known |
|
| Participants | Patients aged ≥ 65 years admitted to 4 wards, including surgical Number of patients recruited: T = 135, C = 138 Mean age: T = 76.5 years, C = 76.6 years |
|
| Interventions |
Setting: hospital in suburb of Copenhagen, Denmark Pre‐admission assessment: no Case finding on admission: not reported; intervention implemented at the time of admission. Inpatient assessment and preparation of a discharge plan based on individual patient needs: patients had daily contact with the project nurse who discussed their illness with them and discharge arrangements Implementation of the discharge plan: there was liaison between hospital and primary care staff. Project nurse visited patients at home after discharge and could make one repeat visit. Monitoring: not reported Control: described as usual care |
|
| Outcomes | Hospital length of stay, readmission to hospital, discharge destination | |
| Notes |
Funding: not known Conflicts of interest: not known Ethical approval: not known Notes: this study was translated from Danish for the first version of this review, in 1997. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Baseline outcome data | Low risk | Comment: Baseline outcome data reported |
| Baseline characteristics similar | Low risk | Comment: Baseline data reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Yes, for objective outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Not reported |
| Study adequately protected against contamination | Unclear risk | Comment: Not reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not reported |
| Other bias | Unclear risk | Comment: Not reported |
Jack 2009.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between January 2006 and October 2007 |
|
| Participants | Patients who were emergency admissions to the medical teaching service and who were going to be discharged home. Participants had to have a telephone, comprehend the study details and consent process in English and have plans to be discharged to a USA community. Number of participants recruited: T = 373, C = 376 Mean age (SD): T: 50.1 (15.1), C: 49.6 (15.3) Sex (female): T = 178/373 (48%), C = 200/376 ( 53%) |
|
| Interventions |
Setting: large urban safety net hospital with an ethnically diverse patient population; Boston Medical Centre, Massachusetts, USA Pre‐admission assessment: no Case finding on admission: the nurse discharge advocate (DA) completed the (re‐engineered discharge) RED intervention components Inpatient assessment and preparation of a discharge plan based on individual patient needs: with information collected from the hospital team and the participant, the DA created the after‐hospital care plan (AHCP), which contained medical provider contact information, dates for appointments and tests, an appointment calendar, a colour‐coded medication schedule, a list of tests with pending results at discharge, an illustrated description of the discharge diagnosis, and information about what to do if a problem arises. Information for the AHCP was manually entered into a Microsoft Word template, printed, and spiral‐bound to produce an individualised, colour booklet Implementation of the discharge plan: the DA used scripts from the training manual to review the contents of the AHCP with the participant. On the day of discharge the AHCP and discharge summary were faxed to the primary care provider (PCP). Monitoring phase: clinical pharmacist telephoned the participants 2 to 4 days after the index discharge to reinforce the discharge plan by using a scripted interview. The pharmacist had access to the AHCP and hospital discharge summary and, over several days, made at least 3 attempts to reach each participant. The pharmacist asked participants to bring their medications to the telephone to review them and address medication‐related problems; the pharmacist communicated these issues to the PCP or DA Additional information on the intervention available at www.bu.edu/fammed/projectred/index.html Control: usual care |
|
| Outcomes | Readmission, patient satisfaction and cost at 30 days | |
| Notes |
Funding: Agency for Healthcare Research and Quality and the National Heart, Lung, and Blood Institute, National Institutes of Health, USA Conflicts of interest: first author reported receiving grants from governmental organisations Ethical approval: Institutional review board Notes:rReadmission data obtained from the authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Index cards in opaque envelopes randomly arranged |
| Allocation concealment (selection bias) | Low risk | Comment: The authors state that the research assistants could not selectively choose potential participants for enrolment or predict assignment (p.3) |
| Baseline outcome data | Low risk | Comment: Baseline outcome data collected at recruitment for previous hospital admissions (Table 2) |
| Baseline characteristics similar | Low risk | Comment: Baseline characteristics collected at recruitment (Table 2) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Research staff doing follow‐up telephone calls and reviewing hospital records were blinded to study group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Follow‐up at 30 d > 80%. Similar proportion in both groups |
| Study adequately protected against contamination | Low risk | Comment: Participants recruited from the same centre and allocated to intervention and comparison groups; study personnel delivered the intervention, the control group did not have access to the intervention |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not able to judge from available information |
| Other bias | Low risk | Comment: Not reported |
Kennedy 1987.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between September and October 1984 |
|
| Participants | Elderly acute care medical patients Number of patients recruited: T = 39, C = 41 Mean age: T = 80.1 years, C = 80.5 years Sex (female): T = 19/39 (49%), C = 23/41 (56%) |
|
| Interventions |
Setting: 500‐bed, non‐profit acute care teaching hospital, Texas, USA Pre‐admission assessment: no Case finding on admission: not reported Inpatient assessment and preparation of a discharge plan based on individual patient needs: discharge planning emphasised communication with the patient and family. A primary nurse assessed patients' postdischarge needs. A comprehensive discharge planning protocol was developed, which included an assessment of health status, orientation level, knowledge and perception of health status, pattern of resource use, functional status, skill level, motivation, and demographic data. Implementation of the discharge plan: by the primary nurse and other members of the healthcare team. A follow‐up visit was made to assess discharge placement. Monitoring: not reported Control: care not described |
|
| Outcomes | Hospital length of stay, re‐admission to hospital, discharge destination, health status (8 weeks post‐discharge) | |
| Notes |
Funding: Scott and White Memorial Hospital Conflicts of interest: not reported Ethical approval: not reported Notes: not clear when intervention implemented |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Random number schedule described |
| Allocation concealment (selection bias) | Low risk | Comment: Allocation provided by the statistics department |
| Baseline outcome data | Low risk | Comment: Main outcome is length of stay |
| Baseline characteristics similar | Low risk | Comment: Baseline data reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: For objective measures of outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All patients randomised accounted for at follow‐up |
| Study adequately protected against contamination | Low risk | Comment: No evidence of contamination |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not able to judge from available information |
| Other bias | Low risk | Comment: Not reported |
Kripalani 2012.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between May 2008 and September 2009 |
|
| Participants | Patients hospitalised for acute coronary syndrome or acute decompensated HF, English‐ or Spanish‐speaking, expected to stay in hospital for more than 3 hours, likely to be discharged home, without dementia, active psychosis, bipolar disorder or delirium, without hearing or vision impairment Number recruited: T = 423, C = 428 Mean age (SD): T = 61 years (14.4), C = 59 years (13.8) Sex (female): T = 173/423 (41%), C = 179/428 (42%) |
|
| Interventions |
Setting: tertiary care academic hospitals, Nashville and Boston, USA Pre‐admission assessment: no Case finding on admission: not reported Inpatient assessment and preparation of a discharge plan based on individual patient needs: at the first meeting, the pharmacist assessed the patient's understanding and needs, communicating with the treating physician if medication discrepancies were identified Implementation of the discharge plan: second meeting occurred before discharge and patient was given tailored counselling and low‐literacy adherence aids; if discharge occurred same day as enrolment, then single session was conducted for assessment and implementation of discharge plan. Monitoring: call 1 to 4 days after discharge by unblinded research assistant; if outstanding needs identified, pharmacist would perform follow‐up call, liaising with in‐ and outpatient physician if necessary Control: physicians and nurses performed medication reconciliation and provided discharge counselling; medication reconciliation was facilitated by electronic records. At one of the sites there were additional features (reminders to complete a preadmission medication list and integration with order entry) |
|
| Outcomes | Number of clinically important medication errors at 30 days (composite measure of preventable or ameliorable ADEs and potential ADEs due to medication discrepancies or non‐adherence); preventable or ameliorable ADEs; potential ADEs due to medication discrepancies or non‐adherence; preventable or ameliorable ADEs judged to be serious, life‐threatening, or fatal. | |
| Notes |
Funding: National Heart, Lung, and Blood Institute Conflicts of interest: quote: "Dr. Kripalani [lead author] is a consultant to and holds equity in Bioscape Digital/PictureRx, which makes materials for patient engagement and education. The company’s products and services were not used in this study. all other authors declare no potential conflicts of interest" Ethical approval: University Institutional Review Board and the Partners Human Research Committee |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Randomisation was stratified by study site and diagnosis, in permuted blocks of 2‐6 patients, using a computer programme |
| Allocation concealment (selection bias) | Low risk | Comment: One unblinded research coordinator at each site administered the randomisation using a computer programme that maintained allocation concealment, contacted study pharmacists who then delivered the intervention to eligible patients, and participated in the individualised telephone follow‐up |
| Baseline outcome data | Low risk | Comment: Baseline outcome data provided for median pre‐admission medications and comorbid conditions, and similar between groups (Table 1) |
| Baseline characteristics similar | Low risk | Comment: Participants allocated to the intervention group were slightly older, groups similar other than that (Table 1) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Main outcome determined by 2 independent clinicians following standardised validated methodology, blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Follow‐up at 30 d for > 80%; similar % of drop‐outs in both groups |
| Study adequately protected against contamination | Low risk | Comment: The intervention was delivered by the study pharmacists, who did not have contact with participants allocated to the comparison group. |
| Selective reporting (reporting bias) | Low risk | Comment: Slight discrepancies between protocol and publication, for secondary outcomes and 1 minor inclusion criterion |
| Other bias | Unclear risk | Comment: Not reported |
Lainscak 2013.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between November 2009 and December 2011 |
|
| Participants | Patients admitted with COPD exacerbation with reduced pulmonary function, aged ≥ 35 years, not at terminal stages of disease Number recruited: T = 118, C = 135 Mean age (SD): T = 71 years (9), C = 71 years (9) Sex (female): T = 37/118 (31%), C = 34/135 (25%) Living alone: T = 29 (25%), C = 27 (20%) |
|
| Interventions |
Setting: specialised pulmonary hospital, Slovenia Pre‐admission assessment: no Case finding on admission: not reported Inpatient assessment and preparation of a discharge plan based on individual patient needs: the discharge co‐ordinator assessed patient and home care needs, involving both the patient and the caregiver. Implementation of the discharge plan: within 48 hours of admission the discharge co‐ordinator communicated the discharge plan to PCP, community nurses, and other providers of home services, as required by the patient's needs. Monitoring: phone call at 48 hours postdischarge to assess the adjustment process, followed by phone calls scheduled as required until a final home visit at 7 to 10 days postdischarge Control: care as usual, which included routine patient education with written and verbal information about COPD, supervised inhaler use, respiratory physiotherapy as indicated, and disease related communication between medical staff with patients and their caregivers. |
|
| Outcomes | Number of patients hospitalised due to worsening COPD, time to COPD hospitalisation, all‐cause mortality, all‐cause hospitalisation, days alive and out of hospital, health‐related quality of life (90 days) | |
| Notes |
Funding: no financial support was received for the trial Conflicts of interest: none reported Ethical approval: National Medical Ethics Committee of the Republic of Slovenia Notes: steering and endpoint committee closed enrolment at 83% of the planned sample due to re‐hospitalisation of patients already assessed for eligibility and seasonal variation of COPD. Information about the communication between discharge co‐ordinators and providers of home services, including timing and frequency, was not reported in detail. The authors provided supplementary unpublished data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Software to generate random numbers/allocation sequence (p.450) |
| Allocation concealment (selection bias) | Low risk | Comment: Allocation independent of researchers and healthcare providers (p.450) |
| Baseline outcome data | Low risk | Quote: "The 2 groups of patients were similar with respect to baseline characteristics, disease severity, clinical presentation, comorbidity, and the use of medication at the time of enrolment" (p.450.e3) |
| Baseline characteristics similar | Low risk | Quote: "The 2 groups of patients were similar with respect to baseline characteristics, disease severity, clinical presentation, comorbidity, and the use of medication at the time of enrolment" (p.450.e3) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Objective measure for primary outcome; two physicians unrelated to the study adjudicated whether the patient was hospitalised because of worsening COPD (p450.e2) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Follow‐up at 180 d for > 80%; similar % of drop‐outs in both groups (p.450.e3) |
| Study adequately protected against contamination | Unclear risk | Comment: Patients were allocated within a hospital and it is possible that communication between intervention and control professionals could have occurred |
| Selective reporting (reporting bias) | Low risk | Comment: One of the secondary outcomes not reported (healthcare costs), all other outcomes reported |
| Other bias | Low risk | Comment: Not reported |
Laramee 2003.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between July 1999 and April 2001 |
|
| Participants | Patients with confirmed congestive heart failure (CHF), who also had to be at risk for early readmission as defined by the presence of 1 or more of the following criteria: history of CHF, documented knowledge deficits of treatment plan or disease process, potential or ongoing lack of adherence to treatment plan, previous CHF hospital admission, living alone, and ≥ 4 hospitalisations in the past 5 years Number recruited: T = 141, C = 146 Mean age (SD): T = 70.6 years (11.4), C = 70.8 years (12.2) Sex (female) T = 59/141 (42%), C = 72/146 (50%) Support at home: T = 127/141 (90%), C = 140/146 (96%) |
|
| Interventions |
Setting: 550‐bed academic medical centre, which serves the largely rural geographic areas of Vermont and upstate New York, USA Pre‐admission assessment: no Case finding on admission: no Inpatient assessment and preparation of a discharge plan based on individual patient needs: early discharge planning and co‐ordination of care and individualised and comprehensive patient and family education Implementation of the discharge plan: case manager (CM) assisted in the co‐ordination of care by facilitating the discharge plan and obtaining needed consultations from social services, dietary services and physical/occupational therapy. When indicated, arrangements were made for additional services or support once the patient had returned home. The CM also facilitated communication in the hospital among the patient and family, attending physician, cardiology team, and other medical care practitioners through participating in daily rounds, documenting patient needs in the medical record, submitting progress reports to the PCP, involving the patient and family in developing the plan of care, collaborating with the home health agencies and providing informational and emotional support to the patient and family. Monitoring: 12 weeks of enhanced telephone follow‐up and surveillance Control: inpatient treatments included social service evaluation (25% for usual care group), dietary consultation (15% usual care), PT/OT (17% usual care), medication and CHF education by staff nurses and any other hospital services. Postdischarge care was conducted by the patient's own local physician. The home care service figures were 44%. |
|
| Outcomes | Readmissions, mortality, hospital bed days, resource use and patient satisfaction. Follow‐up at 3 months. | |
| Notes |
Funding: University of Vermont General Clinical Research Center, USA. Novartis Pharmaceuticals. Conflicts of interest: not reported Ethical approval: Institutional review board |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: 'after simple randomzation of the first 42 patients resulted in a large amount of patients being assigned to one group or the other, patients were randomized in blocks of 8 to ensure an even group allocation across time' (page 810). |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Baseline outcome data | Unclear risk | Comment: Some baseline imbalances. Participants allocated to the intervention had more risk factors for readmission, and a higher percentage were assessed as mild on the New York Heart Association classifaction (class ii ‐ mild symptoms and slight limitation during ordinary activity) (Table 1) |
| Baseline characteristics similar | Unclear risk | Comment: Baseline data reported, a higher percentage of participants in the intervention group were assessed as mild on the New York Heart Failure classification (class ii) (Table 1) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Objective measure of the primary outcome readmission, and the secondary outcome length of stay |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Loss to follow‐up: 53/287; ≥ 81% retained. T = 122/141; C = 112/146 |
| Study adequately protected against contamination | Unclear risk | Comment: Participants recruited from the same hospital and allocated to intervention and comparison groups; intervention was delivered by study personnel (p.810, 2nd column) |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not able to judge from available information |
| Other bias | Low risk | Comment: Not reported |
Legrain 2011.
| Study characteristics | ||
| Methods | Randomised trial Investigators used the double consent of a Zelen randomised consent design after assessing patients for eligibility; informed consent was obtained following randomisation. Study conducted between April 2007 and October 2008 |
|
| Participants | Medical patients aged ≥ 70 years; patients were excluded if expected to be discharged in less than 5 days, had poor chance of 3‐month survival or were receiving palliative care Mean age (SD): T = 85.8 years (6.0); C = 86.4 years (6.3) Sex (female): T = 221/317 (70%); C = 218/348 (63%) Number of patients randomised using Zelen design: T = 528; C = 517 (total 1,045) and of these T = 317 and C = 348 participated in the randomised trial |
|
| Interventions |
Setting: 5 university‐affiliated hospitals and 1 private clinic; Paris, France Pre‐admission assessment: not possible Case finding on admission: the intervention focused on 3 risk factors: drug related problems, under‐diagnosis and untreated depression (screened with the 4‐item Geriatric Depression Scale, and if the DSM‐IV criteria were positive) and protein energy malnutrition Inpatient assessment and preparation of a discharge plan based on individual patient needs: the intervention was implemented after admission to the acute geriatric unit (AGU) and had 3 components, a comprehensive chronic medication review according to geriatric prescribing principles and which involved the patient and their caregiver, education on self‐management of disease and detailed transition of care communication with outpatient health professionals and the GP. These were adapted from disease management programmes for inpatients with multiple chronic conditions. Implementation of the discharge plan: the intervention was implemented by a dedicated geriatrician in addition to the care provided by the usual geriatrician of the AGU. The dedicated geriatrician provided recommendations to the AGU geriatrician who made final decisions. GPs were contacted regarding changes in treatment. Monitoring: follow‐up by a geriatrician. Control: received standard medical care from the AGU healthcare team without involvement of the intervention‐dedicated geriatrician. AGUs are hospital units with their own physical location and structure that are specialised in the care of elderly people with acute medical disorders, including acute exacerbations of chronic diseases. AGUs implement comprehensive geriatric assessment. |
|
| Outcomes | Emergency hospitalisation, emergency room visit, mortality, cost Follow‐up time: 6 months from discharge |
|
| Notes |
Funding: Ministry of Health, France Conflicts of interest: none reported Ethical approval: Institutional review board Study stopped early due to service demands and lack of funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Computer‐generated randomisation scheme in various sized blocks stratified according to centre; Zelen study design |
| Allocation concealment (selection bias) | Low risk | Comment: A central randomisation service in the trial organisation centre |
| Baseline outcome data | Low risk | Comment: Outcome data refer to post‐discharge events (readmission, ED visits) |
| Baseline characteristics similar | Low risk | Comment: Majority of baseline characteristics similar between groups |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Objective measure of the primary outcome of readmission and secondary outcome of costs using hospital days. Data on readmission rates were verified by checking administrative databases. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Outcome data reported for all participants recruited |
| Study adequately protected against contamination | Low risk | Comment: Participants recruited from five sites and allocated to intervention or comparison group; dedicated geriatricians delivered the intervention |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not enough information to make a judgement |
| Other bias | Unclear risk | Comment: Zelen study design (p.2026) 1,045 were randomized, and 665 (63%) were included in the study: 317 in the IG and 348 in the CG (Figure 1) |
Lin 2009.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between November 2005 and December 2006 |
|
| Participants | Patients hospitalised with a hip fracture, aged ≥ 65 years, who had a Barthel score of at least 70 points prior to their hip fracture. Number of patients recruited: T = 26; C = 24 Sex (female): 18/50 (36%) Mean age (SD): 78.8 years (7.0) |
|
| Interventions |
Setting: 4 orthopaedic wards in a 2800 bed medical centre in Taipei, Taiwan Pre‐admission assessment: no Case finding on admission: no Inpatient assessment and preparation of a discharge plan based on individual patient needs: structured assessment of discharge planning needs within 48h of admission; systematic individualised nursing instruction based on the individual's needs. Implementation of the discharge plan: nurses coordinated resources and arranged referral placements. Two postdischarge home visits were conducted to provide support and consultation Monitoring: nurses monitored services Control: non‐structured discharge planning provided by nurses who used their professional judgement. |
|
| Outcomes | Hospital length of stay, readmission, functional status, quality of life, patient satisfaction at 2 weeks and 3 months postdischarge | |
| Notes |
Funding: National Science Council, Taiwan Conflicts of interest: not reported Ethical approval: Institutional Review Board |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Patients were assigned to 1 of 4 wards: 2 were designated the intervention group and 2 the control. The sequence generation of random assignment was not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Patients were assigned to 1 of 4 wards "by doctors who were not aware of the study process." |
| Baseline outcome data | Low risk | Comment: Baseline outcome data provided and similar for functional status, quality of life and patient satisfaction (p.1635) |
| Baseline characteristics similar | Low risk | Comment: Similar characteristics at baseline |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: Blinding of researchers conducted follow‐up assessments is not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Data collected on all recruited patients |
| Study adequately protected against contamination | Low risk | Comment: Intervention and comparison groups were in different wards; intervention was delivered by study personnel (p.1634) |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not able to judge from available information |
| Other bias | Low risk | Comment: Not reported |
Lindpaintner 2013.
| Study characteristics | ||
| Methods | Pilot parallel randomised trial Participants recruited between September 2008 and December 2009 |
|
| Participants | Patients aged ≥ 18 years who had been admitted to an internal medicine ward, taking oral anticoagulation or newly ordered insulin or more than 8 regular medicines or new diagnosis requiring at least 4 long‐term medicines, expected to live > 1 month, German‐speaking, no cognitive impairment; excluded if PCP or local visiting nurse association not involved in the study Number of patients recruited: T = 30, C = 30 Mean age (SD): T = 75.1 years (9.49), C = 75.2 (12.4) Sex (female): T = 15/30 (50%), C = 19/30 (63%) |
|
| Interventions |
Setting: teaching hospital in Baden, Switzerland Pre‐admission assessment: no Case finding on admission: all patients admitted to hospital were screened for eligibility Inpatient assessment and preparation of a discharge plan based on individual patient needs: the nurse care manager assessed patients with a battery of tests Implementation of the discharge plan: the NCM liaised with the ward team and jointly developed a discharge plan, which included self‐management techniques; the PCP and community nursing team received a copy of the discharge form, as well as a letter at the end of the intervention, and further contacts were done as needed Monitoring: structured call 24 hours post‐discharge and home visit at the end of the intervention Control: best usual care (no additional information provided) |
|
| Outcomes | Composite endpoint (death, re‐hospitalisation, unplanned urgent medical evaluation within 5 days and 30 days of discharge, and adverse medicine reaction requiring discontinuation of the medicine), satisfaction with discharge process, caregiver burden, health‐related quality of life. The study authors commented that: "the definitions for two components of the primary composite endpoint failed to discriminate sufficiently between adverse events and desirable medical management. Thus planned rehospitalizations and all medicine changes (such as changing a blood pressure medicine) were counted as adverse events even if they reflected medical management decisions unrelated to patient harm." (p.761, 1st column) |
|
| Notes |
Funding: MediService AG, Zuchwil, Switzerland Conflicts of interest: none reported Ethical approval: Internal Review Board Notes: pilot study; insufficient data to be included in the pooled analysis, authors contacted but no further data obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Block randomisation (p.757) |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not reported |
| Baseline outcome data | Low risk | Comment: primary composite outcome of death, rehospitalisation, unplanned urgent medical evaluation within 5 days of discharge and adverse medicine reaction requiring dicontinuation of the medicine. |
| Baseline characteristics similar | Low risk | Comment: 3 patients allocated to the intervention group were receiving ongoing chemotherapy. A small study of 30 in each group. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: Interview‐based data (patients, nurses, and PCP) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Drop‐outs accounted for, intention‐to‐treat analysis |
| Study adequately protected against contamination | High risk | Comment: The same team of physicians and nurses provided inpatient care to both groups (p.759) |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not able to judge from available information |
| Other bias | Unclear risk | Comment: Not reported |
Lisby 2019.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between November 2014 and December 2015 |
|
| Participants | Patients aged >=18 years admitted to the AMU with non‐surgical medical conditions, with at least one hospitalisation in the past 12 months, living in the catchment area and eligible for post‐discharge follow‐up. Patients were excluded if they were deaf or blind, unable to provide consent, and being discharged to destinations other than a private home Number of patients recruited: T = 101, C = 99 Mean age (SD): T = 60.3 years (19.8), C = 61.7 (20.6) Sex (female): T = 42/101 (42%), C = 45/99 (45%) Patients had on average one co‐morbidity, with a relatively low Charlson's Comorbidity score |
|
| Interventions |
Setting: 34‐bed acute medical unit affiliated with the emergency department at Aarhus University Hospital, Denmark Pre‐admission assessment: Case finding on admission: the research nurse or project investigator checked the electronic dashboard for potential eligible patients; the dashboard contained real‐time information on the patient's clinical status, diagnostic procedures and expected discharge Inpatient assessment and preparation of a discharge plan based on individual patient needs: patient's needs were assessed through an algorithm purposely developed for the study, covering ability to manage at home and available help if required, medication, network, other medical needs, and gait, hearing and vision. Any outstanding needs were subsequently addressed by the nurse prior to discharge, including any arrangements for customised aids, if necessary. The nurse also assessed to which extent the patient understood discharge instructions provided by the physician. Implementation of the discharge plan: The patient was sent a detailed discharge letter adapted to their health literacy level, covering admission, type and results of tests performed and further tests required, treatment while in hospital and further treatment required, and contact information for the research team. The PCP also received a copy of the letter. Monitoring: follow‐up call 2 days after discharge Control: triage at admission, measurements of early warning score as prescribed by the physician and an unstructured intake conversation. At discharge the nurse had an unstructured conversation with the patient, who was given an updated medication list, a card with AMU contacts, and if relevant disease‐specific pamphlets. A discharge letter was sent to the PCP, which was sometimes shared with the patient. |
|
| Outcomes |
Main outcomes: proportion of all‐cause 30‐ day readmissions, total number of readmissions 30 days post‐discharge Other outcomes: sub‐analyses of readmissions (72‐hour readmissions, readmissions between 4:00 p.m. and 8:00 a.m., time to first readmission and number of emergency department contacts); preventability of the first readmissions in the follow‐up period |
|
| Notes |
Funding: The Danish Regions and the Danish Health Confederation and the Danish Nurses Organisation Conflicts of interest: none reported Ethical approval: Regional Scientific Ethics Committee of the Central Denmark Region and National Data Protection Agency Notes: Some outcomes were assessed both as per protocol and intention‐to‐treat analyses. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomisation was generated by a specific web‐based program (Trial Partner) in random blocks of 20.” (p.4, 2nd column) |
| Allocation concealment (selection bias) | Unclear risk | Comment: not enough information provided to make a judgment |
| Baseline outcome data | Low risk | Comment: groups were similar for acute medical unit length of stay (Table 1) |
| Baseline characteristics similar | Low risk | Comment: baseline characteristics provided and similar between groups (Table 1) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: due to the nature of the intervention, it was not possible to blind participants or personnel. Objective main outcomes, however not clear if outcome assessors were blinded to group allocation (p.4, 1st column) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: drop outs higher for IG (15%) than CG (6%), reasons explained; ITT and per‐protocol analyses |
| Study adequately protected against contamination | Unclear risk | Comment: group allocation done by participant, who were all in the same acute medical unit |
| Selective reporting (reporting bias) | Low risk | Comment: same outcomes reported in trial registry and publication |
| Other bias | Unclear risk | Quote: “After one year of recruitment, less than half of the required study sample was included and the study was terminated due to futility.” (p.5, 1st column) |
Moher 1992.
| Study characteristics | ||
| Methods | Parallel randomised trial Participants recruited between July and October 1990 |
|
| Participants | Patients admitted to a general medical clinic, excluded if admitted to intensive care unit or not expected to survive for more than 48 hours Number of patients recruited: T = 136, C = 131 Mean age: T = 66.3 years, C = 64.3 years Sex (female): T = 73/136 (54%), C = 72/131 (55%) |
|
| Interventions | Setting: 2 clinical teaching units, Ottawa, Canada Pre‐admission assessment: no Case finding on admission: no Inpatient assessment and preparation of a discharge plan based on individual patient needs: a nurse employed as a team co‐ordinator acted as a liaison between members of the medical team and collected patient information Implementation of the discharge plan: the nurse facilitated discharge planning Monitoring: not reported Control: standard medical care |
|
| Outcomes | Hospital length of stay, readmission to hospital, discharge destination, patient satisfaction. Follow‐up 2 weeks |
|
| Notes |
Funding: Ontario Ministry of Health, Canada Conflicts of interest: not reported Ethical approval: Research Ethics Committee Notes: baseline data recorded only on age, sex, diagnosis. Not clear when intervention implemented |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Computer‐generated blocks |
| Allocation concealment (selection bias) | Unclear risk | Comment: Allocation procedure not described |
| Baseline outcome data | Low risk | Comment: Main outcome was length of stay |
| Baseline characteristics similar | Low risk | Comment: Baseline data reported (Table 2) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Yes for objective measures of outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All patients randomised accounted for at follow‐up |
| Study adequately protected against contamination | Unclear risk | Comment: Not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not able to judge from available information |
| Other bias | Low risk | Comment: No additional sources of bias |
Naji 1999.
| Study characteristics | ||
| Methods | Parallel randomised trial Study dates: not known |
|
| Participants | Patients admitted to an acute psychiatric ward; patients were excluded if previously admitted, too ill, not registered with a GP or had no fixed address. Number of patients recruited: T = 168, C = 175 Mean age (SD): T = 40 (12), C = 41 (12.8) Sex (female): T = 83/168 (49%), C = 80/175 (46%) |
|
| Interventions |
Setting: acute psychiatric wards, Aberdeen, Scotland Pre admission assessment: no Case finding on admission: no Inpatient assessment and preparation of a discharge plan based on individual patient need: not clear Implementation of the discharge plan: psychiatrist telephoned GP to discuss patient and make an appointment for the patient to see the GP within 1 week following discharge. A copy of the discharge summary was given to the patient to hand‐deliver to the GP. A copy was also sent by post. Monitoring: no Control: received standard care, patients advised to make an appointment to see their GP and were given a copy of the discharge summary to hand deliver to the GP |
|
| Outcomes | Readmission, mental health status, discharge process, cost. Follow‐up at 1 month for patient assessed outcomes, 6 months for readmissions | |
| Notes |
Funding: not known Conflicts of interest: not known Ethical approval: not known |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Independent computer programme |
| Allocation concealment (selection bias) | Low risk | Comment: Independent to researchers |
| Baseline outcome data | Low risk | Comment: Baseline outcome data reported |
| Baseline characteristics similar | Low risk | Comment: Baseline data collected on day of discharge: baseline completion T = 132/168 (79%), C = 133/175 (76%) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Objective measures used for readmission, consultations and length of stay. Validated standardised patient assessed outcomes also measured. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Less than 80% for patient assessed: 1 month completion T = 106/168 (63%), C = 111/175 (63%) |
| Study adequately protected against contamination | Unclear risk | Comment: Not reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not reported |
Naughton 1994.
| Study characteristics | ||
| Methods | Parallel randomised trial Study dates: April 1st to December 31st 1991 |
|
| Participants | Patients aged ≥ 70 years admitted from emergency department who were not receiving regular care from an attending internist on staff; patients were excluded if admitted to intensive care unit or surgical ward. Number of patients recruited: T = 51, C = 60 Mean age (SD): T = 80.1 years (6.6), C = 80.1 years (6.4) Sex (female): T = 25/51 (49%), C = 38/60 (63%) |
|
| Interventions |
Setting: private, non‐profit, academic medical centre, Chicago, USA Pre‐admission assessment: no Case finding on admission: not clear Inpatient assessment and preparation of a discharge plan based on individual patient needs: a geriatric evaluation and management team (GEM) assessed the patients' mental and physical health status and psychosocial condition to determine level of rehabilitation required and social needs. A geriatrician and social worker were the core team members. Implementation of the discharge plan: implemented at the time of admission; team meetings with the GEM and nurse specialist and physical therapist took place twice a week to discuss patients' medical condition, living situation, family and social supports, and patient and family's understanding of the patient's condition. The social worker was responsible for identifying and co‐ordinating community resources and ensuring the posthospital treatment place was in place at the time of discharge and 2 weeks later. The nurse specialist co‐ordinated the transfer to home healthcare. Patients who did not have a primary care provider received outpatient care at the hospital. Monitoring: not reported Control: received 'usual care' by medical house staff and an attending physician. Social workers and discharge planners were available on request. |
|
| Outcomes | Hospital length of stay, discharge destination, health service costs | |
| Notes |
Funding: Northwestern Memorial Foundation Conflicts of interest: Ethical approval: Institutional Review Board of Northwestern University |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Card indicating assignment to the intervention or control group were placed sequentially in opaque sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Comment: Sealed envelopes provided by admitting clerk |
| Baseline outcome data | Low risk | Comment: Baseline outcome data reported |
| Baseline characteristics similar | Low risk | Comment: Baseline data reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Yes for objective measures of outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 141 patients initially randomised, of these 25 were ineligible and 5 were transferred to surgical services, leaving 111 to be analysed |
| Study adequately protected against contamination | Unclear risk | Comment: Not reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not able to judge from available information |
| Other bias | Unclear risk | Comment: Not reported |
Naylor 1994.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between July 1989 and February 1992 |
|
| Participants | Patients aged ≥ 70 years, admitted to medical ward and cardiac surgery, English‐speaking, alert and orientated at admission, and able to use telephone after discharge. The medical diagnostic related groups were congestive heart failure and angina/myocardial infarction, the surgical were coronary artery bypass graft and cardiac valve replacement Number of patients recruited: T = 140, C = 136 Mean age (SD): 76 years |
|
| Interventions |
Setting: Hospital of the University of Pennsylvania, USA Pre‐admission assessment: no Case finding on admission: not clear Inpatient assessment and preparation of a discharge plan based on individual patient needs: the discharge plan included a comprehensive assessment of the needs of the elderly patient and their caregiver, an education component for the patient and family and interdisciplinary communication regarding discharge status Implementation of the discharge plan: implemented by geriatric nurse specialist and extended from admission to 2 weeks post‐discharge with ongoing evaluation of the effectiveness of the discharge plan Monitoring: not reported Control: received the routine discharge planning available in the hospital |
|
| Outcomes | Hospital length of stay, readmission to hospital, health status, health service costs Follow‐up at 2, 6, and 12 weeks post‐discharge |
|
| Notes |
Funding: National Institute of Nursing Research, USA Conflicts of interest: not reported Ethical approval: not reported Notes: intervention implemented at time of admission |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Baseline outcome data | Low risk | Comment: Baseline outcome data reported for health status and previous rehospitalisations and similar between groups (Table 1) |
| Baseline characteristics similar | Low risk | Comment: Baseline data reported and similar between groups (Table 1) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Yes, for objective measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All patients included in the final sample accounted for |
| Study adequately protected against contamination | Unclear risk | Comment: Participants recruited from the same hospital and allocated to intervention and comparison groups; study personnel delivered the intervention (p.1000) |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not able to judge from available information |
| Other bias | Low risk | Comment: Not reported |
Nazareth 2001.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between June 1995 and March 1997 |
|
| Participants | Patients aged ≥ 75 years, on 4 or more medicines who were discharged from 3 acute wards and 1 long‐stay ward. Each patient had a mean of 3 chronic medical conditions, and was on a mean of 6 drugs (SD 2) at discharge. Number of patients recruited: T = 181, C = 181 Mean age (SD): 84 years (5.2) Sex (female): T = 112/181 (62%), C = 119/181 (66%) |
|
| Interventions |
Setting: three acute and one long‐stay hospital, London, UK Pre‐admission assessment: no Case finding on admission: not clear Inpatient assessment and preparation of a discharge plan based on individual patient needs: a hospital pharmacist assessed patients' medication, rationalised the drug treatment, provided information and liaised with caregiver and community professionals. An aim was to optimise communication between secondary and primary care professionals. Follow‐up visit by community hospital at 7‐14 d after discharge to check medication and intervene if necessary. Subsequent visits arranged if appropriate. Implementation of the discharge plan: a copy of the discharge plan was given to the patient, caregiver, community pharmacist and GP Monitoring: follow‐up in the community by a pharmacist Control: discharged from hospital following standard procedures, which included a letter of discharge to the GP. The pharmacist did not provide a review of medications or follow‐up in the community |
|
| Outcomes | Hospital readmission, mortality, quality of life, client satisfaction, knowledge and adherence to prescribed drugs, consultation with GP Follow‐up at 3 and 6 months |
|
| Notes |
Funding: National Health Service research and development programme, UK Conflicts of interest: not reported Ethical approval: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Computer generated random numbers |
| Allocation concealment (selection bias) | Low risk | Comment: Allocation by independent pharmacist at the health authority's central community pharmacy office |
| Baseline outcome data | Low risk | Comment: Baseline outcome data reported and similar between groups (Table 2) |
| Baseline characteristics similar | Low risk | Comment: Baseline characteristics reported and similar between groups (Table 1) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Blinding of objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: At each follow‐up time the number of deaths and readmissions were accounted for. 2 control patients moved away prior to 6‐month follow‐up |
| Study adequately protected against contamination | Low risk | Comment: The hospital pharmacist who delivered the intervention had no contact with participants allocated to the comparison group |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not able to judge from available information |
| Other bias | Low risk | Comment: Not reported |
Nguyen 2018.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between November 2015 and January 2017 |
|
| Participants | Patients admitted to hospital with acute coronary syndrome (ACS) Number of patients randomised: 166 (T: 79, C: 87); Analysed: 128 (1month follow‐up; T: 68, C: 60) Mean age: 61.2 years (SD 9.6) Sex (female): 46/166 (28%) Other relevant characteristics: the majority of patients had a discharge diagnosis of non‐ST‐segment elevation ACS (75.3%) and more than two co‐morbidities (53.6%) |
|
| Interventions |
Setting: Heart Institute, Ho Chi Minh City, Vietnam Pre‐admission assessment: no Case finding on admission: patients admitted with ACS were screened for eligibility Inpatient assessment and preparation of a discharge plan based on individual patient needs: the first session was held in hospital 1 week before discharge, and delivered in‐person by a pharmacist; it comprised four components (assessment and advice about ACS knowledge; assessment of past experience with medication and tailored advice; medication aids; teach back and addressing concerns). Implementation of the discharge plan: as part of the counselling session the pharmacist provided instructions on how to use medication and schedule telephone calls. Monitoring phase: the second session was held 2 weeks after discharge, and delivered on the phone, addressing medication‐related issues. Control: usual care; patients had their medication dispensed at the hospital pharmacy or at any private pharmacy, and were followed at a public or private healthcare centre as an outpatient. |
|
| Outcomes | Main outcomes: patient adherence (1 month, 3 months) Other outcomes: mortality, hospital readmission (3 months) |
|
| Notes |
Funding: Vietnam International Education Development via the Project of Training Lecturers with Ph.D. Degree for Universities and Colleges Conflicts of interest: "The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest." Ethical approval: the study was approved by the institutional biomedical research ethics committee Trial registry:NCT02787941 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “online random number generator (randomization.com)” (Randomization and Intervention) |
| Allocation concealment (selection bias) | Low risk | Quote: “Investigators who performed patient recruitment had been concealed the sequence until the intervention was assigned.” (Randomization and Intervention) |
| Baseline outcome data | Low risk | Comment: Groups were similar at baseline for medication adherence, HRQoL and comorbidities (Table 1) |
| Baseline characteristics similar | Low risk | Comment: Baseline characteristics presented and similar between groups (Table 1) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: “Outcome assessors were blinded; patients and pharmacists performing interventions could not be blinded due to the nature of the intervention.” (Randomization and Intervention) Comment: main outcome is self‐reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: between 16% (CG) and 22% (IG) of participants allocated were lost to follow‐up for reasons unknown (Fig.1) |
| Study adequately protected against contamination | Unclear risk | Comment: Not enough information to make a decision. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Same outcomes between trial registry and published trial |
| Other bias | Low risk | Comment: Not reported |
Parfrey 1994.
| Study characteristics | ||
| Methods | Parallel randomised trial Not reported when study was conducted |
|
| Participants | Medical and surgical patients, excluded if admitted for short stay or into units with their own discharge process, previously enrolled in the study, confused or intoxicated, and ≥ 85 years. Number of patients recruited: hospital A: T = 421, C = 420; hospital B: T = 375, C = 384 Mean age (SD): hospital A: T = 53 years (19), C = 53 years (18); hospital B: T = 56 years (18), C = 56 years (18) Sex (female): hospital A: T = 188/421 (45%), C = 184/420 (44%); hospital B: T = 217/374 (58%), C = 210/384 (55%) |
|
| Interventions |
Setting: 2 academic hospitals, Newfoundland, Canada Pre‐admission assessment: no Case finding on admission: developed a questionnaire to identify patients requiring discharge planning Inpatient assessment and preparation of a discharge plan based on individual patient needs: assessment was based on the questionnaire which covered the patient's social circumstances at home; if the admission was an emergency admission or a readmission; the use of allied health and community services; mobility and activities of daily living; medical or surgical condition Implementation of the discharge plan: referrals to allied health professionals following completion of the questionnaire for discharge planning Monitoring: not reported Control: did not receive the questionnaire; discharge planning occurred if the discharge planning nurses identified a patient or received a referral |
|
| Outcomes | Hospital length of stay at 6 and 12 months | |
| Notes |
Funding: National Health and Research Development Program, Canada Conflicts of interest: none reported Ethical approval: approval from the Human Investigations Committee of the Faculty of Medicine, Memorial University, University of Newfoundland and the Medical Advisory Committees of the Memorial Hospital and St John's Hospital Newfoundland. Notes: also validated an instrument to assess high‐risk patients. Intervention implemented at time of admission |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Low risk | Comment: Sealed envelopes |
| Baseline outcome data | Low risk | Comment: Not applicable as outcome as hospital length of stay |
| Baseline characteristics similar | Low risk | Comment: Baseline data reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Yes for objective measures of outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All patients randomised accounted for at follow‐up |
| Study adequately protected against contamination | Unclear risk | Comment: Not reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not able to judge from available information |
| Other bias | Unclear risk | Comment: Not reported |
Preen 2005.
| Study characteristics | ||
| Methods | Parallel randomised trial Study dates not reported |
|
| Participants | Patients with chronic obstructive pulmonary disease, cardiovascular disease, or both; patients had to be registered with a PCP and have at least two community care providers. Number of patients recruited: T = 91, C = 98 Mean age (SD): T = 74.8years (6.7), C = 75.4 (7.9) years Sex: (female): T = 57/91 (62%), C = 58/98 (59%) |
|
| Interventions |
Setting: 2 tertiary hospitals in Western Australia Pre‐admission assessment: no Case finding on admission: no Inpatient assessment and preparation of a discharge plan based on individual patient needs: discharge planning was based on the Australian Enhanced Primary Care Initiative and tailored to each patient. The discharge plan was developed 24 to 48 hours prior to discharge. Problems were identified from hospital notes and patient/caregiver consultation, goals were developed and agreed upon with the patient/caregiver based on personal circumstances, and interventions and community service providers were identified who met patient needs and who were accessible and agreeable to the patient. Implementation of the discharge plan: the discharge plan was faxed to the GP and consultation with the GP was scheduled within 7 d post‐discharge. Copies faxed to all service providers identified on the care plan. Monitoring: research nurse followed up if GP did not respond in 24 hours and the GP scheduled a consultation (within 7 days post‐discharge) for patient review Control: patients were discharged under the hospitals' existing processes following standard practice of Western Australia, where all patients have a discharge summary completed, which is copied to their GP |
|
| Outcomes | SF‐12, patient satisfaction and views of the discharge process and GP views of the discharge planning process at 7 days post‐discharge | |
| Notes |
Funding: Western Australian Department of Health Conflicts of interest: not reported Ethical approval: hospital research ethics committees |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Low risk | Comment: Described as an "allocation concealment technique" |
| Baseline outcome data | Low risk | Comment: Baseline outcome data presented and similar between groups (Table 2) |
| Baseline characteristics similar | Low risk | Comment: At discharge from hospital (Table 1) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: Blinding for objective measures of outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 61/189 patients did not return surveys (32% drop‐out), GP 70.4% response rate at 7 d postdischarge |
| Study adequately protected against contamination | Unclear risk | Comment: Participants allocated to intervention and comparison groups within the same wards; intervention delivered by study personnel who did not have contact with those allocated to the comparison group (p.44, 2nd column) |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not able to judge from available information |
| Other bias | Low risk | Comment: Not reported |
Rich 1993.
| Study characteristics | ||
| Methods | Parallel randomised trial Study dates: April 1988 to March 1089 |
|
| Participants | Patients aged 70 years, with heart failure; patients were excluded if at low risk, resided outside the catchment area, discharged to a nursing home or long‐term care facility, had other illnesses likely to result in readmission, denied consent, or other logistic reasons. Number of patients recruited: T = 63, C = 35 Mean age (SD): T = 80.0 years (6.3), C = 77.3 years (6.1) Sex (female): T = 38/63 (60%), C = 20/35 (57%) Ethnicity: number white T = 29/63, C = 20/35 |
|
| Interventions |
Setting: Jewish Hospital at Washington University Medical Centre, USA Pre‐admission assessment: yes Case finding on admission: screened for heart failure and stratified into readmission risk categories Inpatient assessment and preparation of a discharge plan based on individual patient needs: patients were visited daily by RN to discuss CHF using a booklet developed for the trial and assess and discuss medications, providing a medication card with timing and dosing of all drugs; dietary advice was provided by dietician and study nurse, and patients were given a low‐sodium diet. Implementation of the discharge plan: a social care worker and member of the home care team met with patient to facilitate discharge planning and ease transition. Economic, social and transport problems were identified and managed. The home care nurse visited the patient at home within 48 h of hospital discharge and then 3 times in the first week and at regular intervals thereafter; at each visit the teaching materials, medication, and diet and activity guidelines were reinforced, and any new problems were discussed. Monitoring: study nurse contacted patients by phone, and patients were encouraged to call researchers or personal physician with any new problems or questions. Control: all conventional treatments as requested by the patient's attending physician. These included social service evaluation, dietary and medical teaching, home care and all other available hospital services. Control group received study education materials and formal assessment of medications. The social service consultations and home care referrals were lower (29% versus 34%). |
|
| Outcomes | Length of stay, readmission to hospital, readmission days quality of life, cost at 3 months follow‐up | |
| Notes |
Funding: Community Research Grant‐in‐Aid from the American Heart Association, Missouri Affiliate Conflicts of interest: none reported Ethical approval: details not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: 2:1 treatment:control allocated |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Baseline outcome data | Low risk | Comment: Not applicable as main outcome is length of hospital stay |
| Baseline characteristics similar | Low risk | Comment: Baseline data reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: For objective measures of outcome (readmission, mortality) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All patients randomised accounted for at follow‐up |
| Study adequately protected against contamination | Unclear risk | Comment: Participants in the control group did not receive study educational materials or formal medicine review, and fewer home and social service referrals |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not able to judge from available information |
| Other bias | Unclear risk | Comment: Not reported |
Rich 1995.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between July 1990 and June 1994 |
|
| Participants | Patients aged ≥ 70 years, with confirmed heart failure and at least 1 of the following risk factors for early readmission: prior history of heart failure, 4 or more hospitalisations in the preceding 5 years, congestive heart failure precipitated by acute MI or uncontrolled hypertension. Patients were excluded if resided outside catchment area, planned discharge to a long‐term care facility, severe dementia or psychiatric illness, life expectancy of less than 3 months, refused to participate or other logistic reasons. Number recruited: T = 142, C = 140 Mean age (SD): T = 80.1 years (5,9), C = 78.4 years (6.1) Sex (female): T = 96/142 (68%), C = 83/140 (59%) Ethnicity: non‐white 55% Living alone: T = 58/142 (41%), C = 62/140 (44%) |
|
| Interventions |
Setting: Jewish Hospital at Washington University Medical Centre, US Pre‐admission assessment: no Case finding on admission: yes Inpatient assessment and preparation of a discharge plan based on individual patient needs: included using a teaching booklet, individualised dietary assessment and instruction by a dietician with reinforcement by the cardiovascular research nurse, consultation with social services to facilitate discharge planning and care after discharge, assessment of medications by geriatric cardiologist, intensive follow‐up after discharge though the hospital's home care services, plus individualised home visits and telephone contact with the study team. Implementation of the discharge plan: with social services Monitoring: not clear Control: received all standard treatment and services ordered by their primary physicians |
|
| Outcomes | Mortality, readmission to hospital, quality of life, cost at 3 months follow‐up. Quality of life and cost data were collected from a subgroup of patients only: quality of life = 126, cost = 57 | |
| Notes |
Funding: National Heart, Lung, and Blood Institute, USA Conflicts of interest: not reported Ethical approval: Institutional review board |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Low risk | Comment: Neither patient nor members of the study team were aware of the treatment assignment until after randomisation |
| Baseline outcome data | Low risk | Comment: Baseline outcome data provided for quality of life and similar between groups (Table 4) |
| Baseline characteristics similar | Low risk | Comment: Baseline data reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: For objective measures of outcome (mortality, readmissions and death) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All patients randomised accounted for at follow‐up |
| Study adequately protected against contamination | Unclear risk | Comment: Participants allocated to intervention and comparison groups within the same wards; intervention delivered by study personnel who did not have contact with those allocated to the comparison group (p.1191, top 1st column) |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not able to judge from available information |
| Other bias | Low risk | Comment: Not reported |
Shaw 2000.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between August 1995 and February 1996 |
|
| Participants | Patients discharged from a psychiatric hospital or care of the elderly ward; patients were excluded if they were prescribed medication at discharge, received a primary diagnosis of drug or alcohol abuse or dementia, and refused home visits after discharge. Number of patients recruited: T = 51, C = 46 Mean age (SD): 47 (17) Sex (female): 61 (63%) |
|
| Interventions |
Setting: psychiatric hospital in South Glasgow, Scotland Pre‐admission assessment: no Case finding on admission: no Inpatient assessment and preparation of a discharge plan based on individual patient needs: pre‐discharge assessment with a pharmacy checklist which assessed patient's knowledge and identified particular problems, such as therapeutic drug monitoring, compliance aid requirements and side effects Implementation of the discharge plan: a pharmacy discharge plan was supplied to the patients' community pharmacist for the intervention group Monitoring: not clear Control: care not described |
|
| Outcomes | Knowledge about medicines, readmission to hospital, readmission due to non‐compliance, medication problems after being discharged from hospital | |
| Notes |
Funding: Primary Care Development Initiative, Scottish Government Conflicts of interest: not reported Ethical approval: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Table of generated numbers with a randomised permuted block size of 6 |
| Allocation concealment (selection bias) | Low risk | Comment: Randomisation by the project pharmacist |
| Baseline outcome data | Low risk | Comment: Outcomes refer to post‐discharge (readmission) |
| Baseline characteristics similar | Low risk | Comment: Baseline characteristics reported as similar between groups (p.146) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: Details of how data were collected for readmission and and length of stay were not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: > 30% attrition at 12 weeks |
| Study adequately protected against contamination | Unclear risk | Comment: Intervention was delivered by the pharmacist, who did not have contact with participants allocated to the comparison group. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not able to judge from available information |
| Other bias | Low risk | Comment: Not reported |
Sulch 2000.
| Study characteristics | ||
| Methods | Parallel randomised trial Study dates not reported |
|
| Participants | Patients admitted to the acute stroke unit and receiving rehabilitation, with persistent impairment and functional limitations. Patients were excluded if they had mild deficits or premorbid physical or cognitive disability Number recruited: integrated care pathway (ICP) = 76, multidisciplinary team (MDT) = 76 Mean age (SD): ICP = 75 (11) years, MDT = 74 (10) years |
|
| Interventions |
Setting: stroke rehabilitation unit at a teaching hospital in London, UK Pre‐admission assessment: no Case finding on admission: no Inpatient assessment and preparation of a discharge plan based on individual patient needs: rehabilitation and discharge planning, with regular review of discharge plan Implementation of the discharge plan: senior nurse implemented the ICP. Multidisciplinary training preceded implementation of the ICP. ICP was piloted for 3 months prior to recruitment to the trial. Monitoring: not reported Control: multidisciplinary model of care in which patients' progress determined goal setting, rather than short‐term goals being determined in advance. The care received by the control group was reviewed and a 3‐month period of implementation was undertaken to exclude bias caused by a placebo effect of undertaking the trial. Groups received comparable amounts of physiotherapy and occupational therapy. |
|
| Outcomes | Hospital length of stay, discharge destination, mortality at 26 weeks, mortality or institutionalisation, activities of daily living index, anxiety and depression, quality of life | |
| Notes |
Funding: NHS R&D Executive North Thames Research Implementation Committee, UK; NHS Health Technology Assessment grant Conflicts of interest: Not reported Ethical approval: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Computer‐generated list of randomised numbers |
| Allocation concealment (selection bias) | Low risk | Comment: Randomisation office allocated patients to intervention or control |
| Baseline outcome data | Low risk | Comment: Main outcome is length of stay |
| Baseline characteristics similar | Low risk | Comment: Baseline data reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Participants and health professionals aware of allocation group; low risk for objective outcomes (readmission, mortality and length of stay) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All patients randomised accounted for at follow‐up |
| Study adequately protected against contamination | Unclear risk | Comment: Participants randomised to intervention or comparison unit, however same healthcare professionals provided care to both (p.1930) |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not able to judge from available information |
| Other bias | Low risk | Comment: Not reported |
Weinberger 1996.
| Study characteristics | ||
| Methods | Parallel randomised trial Study conducted between November 1992 and July 1994 |
|
| Participants | Patients with diabetes mellitus, HF, COPD; patients were excluded if already receiving care at a primary care clinic, residing or being discharged to nursing home, admitted for surgical procedure or cancer diagnosis, if cognitively impaired and had no caregiver, and if had no access to a telephone. Number of patients recruited: T = 695, C = 701 Mean age (SD): T = 63.0 years (11.1), C = 62.6 years (10.9) Sex (female): T = 7/695 (1%), 14/701 (2%) |
|
| Interventions |
Setting: 9 Veterans Affairs hospitals, USA Pre‐admission assessment: no Case finding on admission: no Inpatient assessment and preparation of a discharge plan based on individual patient needs: 3 days before discharge a primary nurse assessed the patient's post‐discharge needs. 2 days before discharge the primary care physician visited the patient and discussed patient's discharge plan with the hospital physician and reviewed the patient. Primary nurse made an appointment for the patient to visit the primary care clinic within 1 week of discharge. Implementation of the discharge plan: patient provided with education materials and given a card with the names and beeper numbers of the primary care nurse and physician. Primary care nurse telephoned the patient within 2 working days after discharge. Primary care physician and primary nurse reviewed and updated the treatment plan at the 1st post‐discharge appointment. Monitoring: not reported Control: did not have access to the primary care nurse and received no supplementary education or assessment of needs beyond usual care |
|
| Outcomes | Re‐admission to hospital, health status, patient satisfaction, intensity of primary care (6 months follow‐up) | |
| Notes |
Funding: Veterans Affairs Cooperative Study in Health Services No. 8, USA; Career Development Program, USA Conflicts of interest: not reported Ethical approval: Research and Human Subjects Committee Notes: discharge planning within 3 days of discharge. Nine VA hospitals participated in the trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Produced by statistical coordinating centre |
| Allocation concealment (selection bias) | Low risk | Comment: Allocation made by telephoning the statistical coordinating centre |
| Baseline outcome data | Low risk | Comment: Baseline outcome data reported and similar between groups (Table 2) |
| Baseline characteristics similar | Low risk | Comment: Baseline data reported and similar between groups (Table 2) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Objective measures of outcome and telephone interviews |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All patients randomised accounted for at follow‐up |
| Study adequately protected against contamination | Unclear risk | Comment: Participants allocated to intervention and comparison groups within the same wards; intervention delivered by study personnel who did not have contact with those allocated to the comparison group (p.1442) |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not able to judge from available information |
| Other bias | Low risk | Comment: Not reported |
ACS: acute coronary system; ADE: adverse drug event; ADL: activities of daily living; AGU: acute geriatric unit; AHCP: after‐hospital care plan; AHCPR: Agency for Health Care Policy and Research; AMI: acute myocardial infarction; C: control; CHF: congestive heart failure; CM: case manager; COPD: chronic obstructive pulmonary disease; DA: discharge advocate; DC: discharge coordinator; DSM: Diagnostic and Statistical Manual of Mental Disorders; ED: emergency department; GEM: geriatric evaluation and management team; GP: general practitioner; HF: heart failure; HRQoL: health‐related quality of life; IADL: instrumental activities of daily living; ICP: integrated care pathway; MDT: Multidisciplinary team; MI: myocardial infarction; MM: mini‐mental assessment; NCM: nurse care manager; NP: Nurse practitioner; OT: occupational therapist; PCP: primary care provider; PO: Primary outcome; PT: physiotherapist; RA: research assistant; RED: re‐engineered discharge; RN: registered nurse; SD: standard deviation; T: treatment; TIA: transient ischaemic attack.
We added three risk of bias criteria (baseline outcome data, protection against contamination and other bias), which were independently assessed by two reviewers (DCGB and SS). For three trials we were not able to obtain paper or electronic copies (Hendriksen 1990; Naji 1999; Parfrey 1994), and do not report risk of bias for those criteria.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abadi 2017 | Intervention is delivered for 12 weeks post‐discharge |
| Applegate 1990 | Discharge planning plus geriatric assessment unit |
| Borenstein 2016 | Intervention was CGA with redesigned interprofessional team‐based care |
| Brooten 1987 | Discharge planning plus home care package |
| Brooten 1994 | Discharge planning plus home care package plus counselling |
| Casiro 1993 | Discharge planning plus home care package |
| Chen 2017 | Post‐discharge component |
| Choong 2000 | Intervention is clinical pathway for patients with a fractured neck of femur, discharge planning is not described |
| Clemson 2016 | Comparison group also received discharge planning |
| Diplock 2017 | Comparison group also received discharged planning |
| Drummond 2012 | Comparison is not usual care |
| Englander 2014 | Transitional care intervention; the only element of discharge planning was primary care‐medical home linkage |
| Germain 1995 | Geriatric assessment and intervention team |
| González‐Guerrero 2014 | Control group given the same manual as intervention group at discharge |
| Haggmark 1997 | Study design not clear |
| Hegelund 2019 | Intervention delivered at point of discharge |
| Hickey 2000 | Patients in the intervention group received discharge planning from a nurse case manager, patients in the control group received discharge planning on request |
| IRCT2016072119141N2 | Intervention is delivered for 6 months post‐discharge |
| Jenkins 1996 | Intervention is discharge teaching book |
| JPRN‐UMIN000029404 | Comparison group also received discharged planning |
| Karppi 1995 | Discharge planning plus geriatric assessment unit |
| Kempen 2020 | The focus of the intervention was i) pharmacist‐led comprehensive medication review, ii) a pharmacist‐led comprehensive medication review with post‐discharge follow‐up, ii) usual care without a pharmacist |
| Kleinpell 2004 | Intervention and control groups received discharge planning, the intervention group also received a discharge planning questionnaire |
| Lang 2017 | Intervention and control groups received discharge planning |
| Linden 2014 | 1. Multidimensional intervention, based on the transitional care model 2. Control group also received discharge planning |
| Lindhardt 2019 | Intervention was implemented at point of discharge |
| Lisby 2018 | Intervention focuses on the promotion of communication between pharmacist/ pharmacologist/orthopaedic physician |
| Loffler 2014 | Medication review only, not discharge planning |
| Lopes Oscalices 2019 | Intervention focuses on patient education to improve understanding of heart failure and medicines |
| Luo 2019 | Intervention is delivered for 6 months post‐discharge |
| Martin 1994 | Discharge planning plus hospital at home |
| Martin‐Sanchez 2019 | Intervention was implemented at point of discharge |
| Marusic 2013 | Intervention was standardised to all patients; no individual assessment done |
| McGrory 1994 | Assessed primary nursing and discharge teaching |
| McInnes 1999 | Both groups received discharge planning, intervention group also received GP input to discharge planning process |
| Naylor 1999 | Discharge planning and home follow‐up. |
| Naylor 2004 | Complex package of care; main emphasis was not discharge planning |
| NCT02112227 | Intervention starts at post‐discharge; intervention is mainly nurse navigator, not discharge planning |
| NCT02351648 | Intervention is transitional care model |
| NCT03258632 | Intervention is delivered 6 weeks post‐discharge |
| Nickerson 2005 | No results reported for the control group |
| Pourrat 2017 | Intervention focuses on promoting communication between hospital and community pharmacies |
| Puschner 2008 | Post‐discharge component |
| Ravn‐Nielsen 2018 | Main components of the intervention are hospital pharmacist review, adding information to the electronic record, and communicating with the physician. Patient receives a 30‐minute post‐discharge interview. |
| Rich 1993b | Pilot study of discharge planning plus home care package |
| Rich 1995b | Discharge planning plus home care package |
| Saleh 2012 | Post‐discharge care |
| Salmani 2018 | Intervention is mainly educational; post‐discharge component |
| Schnipper 2021 | Stepped wedge randomised design; the intervention evolved during the study |
| Shah 2013 | Intervention was standardised to all patients; no individual assessment done |
| Sharif 2014 | Intervention solely focused on providing education and information |
| Shyu 2010 | Multifaceted intervention which included a home care component |
| Townsend 1988 | Post‐discharge care |
| Tseng 2012 | Intervention included a large component of rehabilitation that was not available to the control group |
| Van Hollebeke 2016 | Intervention evaluated the impact of a hospital‐to‐community pharmacist medication records scheme on post‐discharge continuity of patient treatment. |
| Victor 1988 | Augmented home‐help scheme |
| Voirol 2004 | Intervention was standardised to all patients; no individual assessment done |
| Xu 2019 | Intervention focuses on medication and disease management and secondary prevention |
| Yeung 2012 | Multidimensional intervention, based on the transitional care model |
CGA: comprehensive geriatric assessment; HMO: health maintenance organisation
Characteristics of ongoing studies [ordered by study ID]
DRKS00015996.
| Study name | Vun nix kütt nix ‐ patient, geriatrician and general practitioner as a multiprofessional team for intersectoral discharge management |
| Methods | Parallel randomised trial |
| Participants | Setting: Germany Inclusion criteria: >= 65 years, >= 2 chronic conditions Exclusion criteria: unable to consent, language limitations |
| Interventions | Intervention: comprehensive geriatric assessment, intersectoral discharge management with patient education and family physician contact Comparison: comprehensive geriatric assessment, normal discharge management |
| Outcomes | Main outcome: hospital readmission Other outcomes: length of hospital stay, nursing home use, number of drugs, presence of depression symptoms, measure of activity of the patient, quality of life, self‐efficacy, patient satisfaction, family doctors satisfaction |
| Starting date | October 2019 |
| Contact information | Maria Polidori Nelles (maria.polidori‐nelles@uk‐koeln.de) |
| Notes |
Ergan 2018.
| Study name | Structured discharge and follow‐up protocol for COPD Patients receiving LTOT and NIV |
| Methods | Open ‐abel parallel randomised trial |
| Participants | Setting: Turkey Inclusion criteria: aged 40 to 85 years, diagnosis of COPD, eligible for long‐term oxygen therapy (LTOT) or noninvasive ventilation (NIV) Main exclusion criteria: already receiving long‐term oxygen therapy (LTOT) or noninvasive ventilation (NIV) |
| Interventions | Intervention: structured discharge, including patient and relatives education about disease severity, medication and equipment use; preparation of home environment for patient needs; telephone follow‐up at 7 and 14 days post‐discharge Comparison: usual care |
| Outcomes | Main outcome: hospital readmission at 90 days Other outcomes: time to first exacerbation, rate of exacerbation, rate of hospitalisation, compliance to treatment, survival at 12 months |
| Starting date | November 2016 |
| Contact information | Begum Ergan |
| Notes | Trial registry NCT03499470 Estimated completion date August 2019 |
Grischott 2018.
| Study name | Improving inappropriate medication and information transfer at hospital discharge: a cluster‐RCT |
| Methods | Double‐centre double‐blind cluster‐randomised parallel‐controlled clinical trial |
| Participants | Setting: Switzerland Main inclusion criteria: hospitalised adults aged >=60 years, with >=5 drugs prescribed Main exclusion criteria: life expectancy <3 months; cognitive inability to follow study procedures |
| Interventions | Intervention: at a cluster level, senior health physicians will receive a 2 hours "teach‐the‐teachers" session on how to integrate discharge procedure into their daily practice; at a patient level, junior physicians review the patient's medication list using a checklist, after which they develop an optimised discharge medication plan. Comparison: at a cluster level, senior health physicians will attend a 2 hours session on multimorbidity; patient will be discharged according to usual procedure. |
| Outcomes | Main outcome: number of days until the first readmission to (any) hospital (6 months post‐discharge) Other outcomes: readmission rates; number of ED visits or GP encounters; death; number of drugs at discharge; proportion of potentially inappropriate medications; patients quality of life. Outcomes collected at 1, 3, and 6 months post‐discharge unless otherwise specified |
| Starting date | Start date January 2017 Estimated completion date September 2021 |
| Contact information | Dr Stefan Neuner‐Jehle (stefan.neuner‐jehle@usz.ch) |
| Notes | Trial registry ISRCTN18427377 |
NCT02388711.
| Study name | Comprehensive transitional care program for dementia patients |
| Methods | Single‐blinded parallel randomised trial |
| Participants | Setting: USA Inclusion criteria: aged ≥ 65 years, diagnosis of dementia, informal care giver available for regular contact, English‐speaking, access to telephone Main exclusion criteria: discharged to institutional setting, moderate‐high alcohol intake, other complex health issues |
| Interventions | Intervention: nurse case manager; inpatient meeting before discharge; 1‐4 postdischarge phone calls Control: care as usual |
| Outcomes | Change from baseline in rehospitalisation at 14, 30 and 90 d |
| Starting date | March 2015 |
| Contact information | — |
| Notes | Estimated completion date March 2022 (temporarily suspended due to Covid‐19) ClinicalTrials.gov Identifier: NCT02388711 |
NCT02421133.
| Study name | Transitional care program on 30‐day hospital readmissions for elderly patients discharged from a short stay geriatric ward (PROUST) |
| Methods | Open‐label parallel steppe‐ wedge randomised trial |
| Participants | Setting: acute geriatric service, France Inclusion criteria: aged ≥ 75 years, admitted for > 48 hours, discharged home, at risk of readmission/ER visit Main exclusion criteria: hospital at home, not local |
| Interventions | Intervention: pre‐discharge needs assessment; medication reconciliation; comprehensive discharge summary with medication review; direct communication with primary care team and scheduling of follow‐up appointment within 30 days of discharge; phone call and home visits for 4 weeks postdischarge Control: care as usual |
| Outcomes | Main outcome: unscheduled readmission and emergency room visits rate at 30 days |
| Starting date | May 2015 |
| Contact information | — |
| Notes | Estimated completion date August 2018 ClinicalTrials.gov Identifier: NCT02421133 |
NCT03358771.
| Study name | COPD Discharge bundle delivered alone or enhanced through a care coordinator (PRIHS) |
| Methods | Triple‐blinded cross‐over randomised trial |
| Participants | Setting: Canada Inclusion criteria: aged >=50 years, diagnosed with COPD Main exclusion criteria: diagnosis other than COPD |
| Interventions | Intervention: COPD discharge care bundle and coordinator Active comparator: COPD discharge care bundle Comparison: usual care |
| Outcomes | Main outcomes: emergency room revisits at 30 days, hospital readmissions at 30 days Other outcomes: emergency room revisits (7 days, 6 months, 1 year); hospital readmissions (7 days, 6 months, 1 year); mortality; time to first physician visit; patient experience; economic evaluation |
| Starting date | March 2017 |
| Contact information | Marta Michas (marta.michas@ualberta.ca), Michael K Stickland (michael.stickland@ualberta.ca) |
| Notes | Estimated completion date March 2020 |
NCT03496896.
| Study name | Transition cAre inteRvention tarGeted to High‐risk patiEnts To Reduce rEADmission (TARGET‐READ) |
| Methods | Single‐blinded parallel randomised trial |
| Participants | Setting: Switzerland Inclusion criteria: aged >=18 years, at high risk of 30‐day readmission Main exclusion criteria: no phone access, limited language skills |
| Interventions | Intervention: pre‐discharge component (patient information, medication reconciliation, patient education, planning of a first post‐discharge primary care physician visit with a timely discharge summary sent to the primary care physician); post‐discharge component (two follow‐up phone calls made by a nurse, including assessment of the general health condition and verification of the follow‐up care plan) Comparison: usual care |
| Outcomes | Main outcome: 30‐day unplanned hospital readmission or mortality Other outcomes: 30‐day unplanned hospital readmission; 30‐day mortality; time to first unplanned readmission or mortality; patient's satisfaction; healthcare use; costs |
| Starting date | April 2018 |
| Contact information | Jacques Donzé (jacques.donze@insel.ch) |
| Notes | Estimated completion date March 2020 |
NCT04154917.
| Study name | Effectiveness of a comprehensive patient‐centered hospital discharge planning Intervention for frail older adults (HOME) |
| Methods | Parallel randomised controlled trial |
| Participants | Setting: Canada Inclusion criteria: aged>=70 years, mild cognitive impairment, expected hospital stay >=5 days, expected to return to live in the community after discharge Exclusion criteria: none reported |
| Interventions | Intervention: inpatient needs assessment, pre‐discharge home assessment, follow‐up home visit and phone call Comparison: customary discharge planning assessment |
| Outcomes | Main outcomes: functional autonomy, unplanned hospital readmission Other outcome: goal attainment |
| Starting date | November 2019 |
| Contact information | Natasa Obradovic (mailto:natasa.obradovic%40usherbrooke.ca?subject=NCT04154917, 389430, Effectiveness of a Comprehensive Patient‐centered Hospital Discharge Planning Intervention for Frail Older Adults); Ariane Grenier (mailto:ariane.grenier%40usherbrooke.ca?subject=NCT04154917, 389430, Effectiveness of a Comprehensive Patient‐centered Hospital Discharge Planning Intervention for Frail Older Adults) |
| Notes | Estimated completion date April 2021 |
COPD: chronic obstructive pulmonary disease; RCT: randomised controlled trial
Differences between protocol and review
We updated the methods to comply with the MECIR standards, included a Summary of Findings Table, and assessed the certainty of the evidence with GRADE.
Contributions of authors
Daniela Gonҫalves‐Bradley (DGB): screened abstracts, retrieved and screened full‐texts, extracted data, contributed to the data analysis and approved the final draft of the review.
Natasha Lannin (NL): screened abstracts and full‐texts, approved the final draft of the review.
Lindy Clemson (LC):screened abstracts and full‐texts, approved the final draft of the review.
Ian Cameron (IC): screened abstracts and full‐texts, approved the final draft of the review.
Sasha Shepperd (SS): co‐authored the protocol for the review with Julie Parkes, screened studies for inclusion, extracted and analysed data, and led the revisions and writing of this update.
Sources of support
Internal sources
-
Nuffield Department of Population Health, University of Oxford, UK
Sasha Shepperd
External sources
-
NIHR Evidence Synthesis Award to SS and NHS Cochrane Collaboration Programme Grant Scheme, UK
Support for previous versions of this review
-
NIHR Cochrane Infrastructure funding to the EPOC group, UK
This review was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the EPOC Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the National Institute of Health Research or the Department of Health and Social Care.
Declarations of interest
DGB: none known. NL: none known. LC: none known. IC: none known. SS: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Balaban 2008 {published data only}
- Balaban RB, Weissman JS, Samuel PA, Woolhandler S.Redefining and redesigning hospital discharge to enhance patient care: a randomized controlled study. Journal of General Internal Medicine 2008;23(8):1228-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolas 2004 {published data only}
- Bolas H, Brookes K, Scott M, McElnay J.Evaluation of a hospital-based community liaison pharmacy service in Northern Ireland. Pharmacy World & Science 2004;26(2):114-20. [DOI] [PubMed] [Google Scholar]
Bonetti 2018 {published data only}
- Bonetti AF, Bagatim BQ, Bottacin WE, Mendes AM, Rotta I, Reis RC, et al.Pharmacotherapy problems in cardiology patients 30 days post discharge from a tertiary hospital in Brazil: a randomized controlled trial. Clinics 2019;74:e1091. [DOI: 10.6061/clinics/2019/e1091] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonetti AF, Bagatim BQ, Mendes AM, Rotta I, Reis RC, Favero ML, et al.Impact of discharge medication counseling in the cardiology unit of a tertiary hospital in Brazil: a randomized controlled trial. Clinics 2018;73:e325. [DOI: 10.6061/clinics/2018/e325] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cajanding 2017 {published data only}
- Cajanding RJ.Effects of a structured discharge planning program on perceived functional status, cardiac self-efficacy, patient satisfaction, and unexpected hospital revisits among Filipino cardiac patients: a randomized controlled study. Journal of Cardiovascular Nursing 2017;31(1):67-77. [DOI: 10.1097/JCN.0000000000000303 ] [DOI] [PubMed] [Google Scholar]
Eggink 2010 {published data only}
- Eggink RN, Lenderink AW, Widdershoven JW, Van den Bemt PLM.The effect of a clinical pharmacist discharge service on medication discrepancies in patients with heart failure. Pharmacy World & Science 2010;32(6):759-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 1993 {published data only}
- Evans RL, Hendricks RD.Evaluating hospital discharge planning: a randomised controlled trial. Medical Care 1993;31(4):358-70. [DOI] [PubMed] [Google Scholar]
Farris 2014 {published data only}
- Carter BL, Farris, KB, Abramowitz PW, Weetman DB, Kaboli PJ, Dawson JD, et al.The Iowa Continuity of Care Study: background and methods. American Journal of Health-System Pharmacy 2008;65(17):1631-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley TM, Shelsky C, Powell S, Farris KB, Carter BL.Effect of clinical pharmacist intervention on medication discrepancies following hospital discharge. International Journal of Clinical Pharmacy 2014;36(2):430-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris KB, Carter BL, Xu J, Dawson JD, Shelsky C, Weetman DB, et al.Effect of a care transition intervention by pharmacists: an RCT. BMC Health Services Research 2014;14:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel EN, Farley TM, Farris KB, Carter BL.Underutilization of cardiovascular medications: effect of a continuity-of-care program. American Journal of Health-System Pharmacy 2013;70(18):1592-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillespie 2009 {published data only}
- Gillespie U, Alassaad A, Henrohn D, Garmo H, Hammarlund-Udenaes M, Toss H, et al.A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Archives of Internal Medicine 2009;169(9):849-900. [DOI] [PubMed] [Google Scholar]
Goldman 2014 {published and unpublished data}
- Goldman.Request of extra data for Support From Hospital to Home for Elders [personal communication]. Email to D Gonçalves-Bradley 10 April 2015.
- Goldman LE, Sarkar U, Kessell E, Critchfield J, Schneidermann M, Pierluissi E, et al.Support for hospital to home for elders: a randomized control trial of an in-patient discharge intervention among a diverse elderly population. Journal of General Internal Medicine 2013;28(Supplement 1):S189-S190.
- Goldman LE, Sarkar U, Kessell E, Guzman D, Schneidermann M, Pierluissi E, et al.Support from hospital to home for elders: a randomized trial. Annals of Internal Medicine 2014;161(7):472-81. [DOI] [PubMed] [Google Scholar]
- Greysen SR, Hoi-Cheung D, Garcia V, Kessell E, Sarkar U, Goldman L, et al."Missing pieces"--functional, social, and environmental barriers to recovery for vulnerable older adults transitioning from hospital to home. Journal of the American Geriatrics Society 2014;62(8):1556-61. [DOI: 10.1111/jgs.12928] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harrison 2002 {published data only}
- Harrison MB, Browne GB, Roberts J, Tugwell P, Gafni A, Graham I.Quality of life of the effectiveness of two models of hospital-to-home transition. Medical Care 2002;40(4):271-82. [DOI] [PubMed] [Google Scholar]
Hendriksen 1990 {published data only}
- Hendriksen C, Strømgård E, Sørensen KH.Cooperation concerning admission to and discharge of elderly people from the hospital. 1. The coordinated contributions of home care personnel [Samarbejde om gamle menneskers sygehusindlaeggelse og - udskrivelse. 1. Hjemmesygeplejerskens koordinerende indsats pa sygehuset]. Ugeskrift For Laeger 1989;151(24):1531-4. [PubMed] [Google Scholar]
- Hendriksen C, Stromgard E, Sorensen K.Current cooperation concerning admission to and discharge from geriatric hospitals [Nyt samarbejde om gamle menneskers syehusindlaeggelse og - udskrivelse]. Nordisk Medicin 1990;105(2):58-60. [PubMed] [Google Scholar]
Jack 2009 {published data only}
- Jack BW, Chetty VK, Anthony D, Greenwald JL, Sanchez GM, Johnson AE, et al.A reengineered hospital discharge program to decrease rehospitalization. Annals of Internal Medicine 2009;150(3):178-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kennedy 1987 {published data only}
- Kennedy L, Neidlinger S, Scroggins K.Effective comprehensive discharge planning. Gerontologist 1987;27(5):577-80. [DOI] [PubMed] [Google Scholar]
Kripalani 2012 {published data only}
- Bell SP, Schnipper JL, Goggins K, Bian A, Shintani A, Roumie C L, et al Pharmacist Intervention for Low Literacy in Cardiovascular Disease Study, Group.Effect of pharmacist counseling intervention on health care utilization following hospital discharge: a randomized control trial. Journal of General Internal Medicine 2016;31(5):470-7. [DOI: 10.1007/s11606-016-3596-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SP, Schnipper JL, Goggins KM, Bian A, Shintani A, Roumie CL, et al.Effect of a pharmacist counseling intervention on healthcare utilization after hospital discharge: a randomized controlled trial. Journal of General Internal Medicine 2015;30(Supplement 2):S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripalani S, Roumie CL, Dalal AK, Cawthon C, Businger A, Eden SK, et al, PILL-CVD (Pharmacist Intervention for Low Literacy in Cardiovascular Disease) Study Group.Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Annals of Internal Medicine 2012;157(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnipper JL, Roumie CL, Cawthon C, Businger A, Dalal AK, Mugalla I, et al, PILL-CVD Study Group.Rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL-CVD) study. Circulation: Cardiovascular Quality and Outcomes 2010;3(2):212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lainscak 2013 {published and unpublished data}
- Farkas J, Kadivec S, Kosnik M, Lainscak M.Effectiveness of discharge-coordinator intervention in patients with chronic obstructive pulmonary disease: study protocol of a randomized controlled clinical trial. Respiratory Medicine 2011;105(Suppl 1):S26-S30. [DOI] [PubMed] [Google Scholar]
- Lainscak M, Kadivec S, Kosnik M, Benedik B, Bratkovic M, Jakhel T, et al.Discharge coordinator intervention prevents hospitalisations in patients with COPD: a randomized controlled trial. European Respiratory Journal 2012;40(S56):P2895. [DOI] [PubMed] [Google Scholar]
- Lainscak M, Kadivec S, Kosnik M, Benedik B, Bratkovic M, Jakhel T, et al.Discharge coordinator intervention prevents hospitalizations in patients with COPD: a randomized controlled trial. Journal of the American Medical Directors Association 2013;14(6):450.e1-6. [DOI] [PubMed] [Google Scholar]
- Lainscak M.Request of extra data for "Discharge Coordinator intervention" [personal communication]. Email sent to D Gonçalves-Bradley 15 April 2015.
Laramee 2003 {published data only}
- Laramee AS, Levinsky SK, Sargent J, Ross R, Callas P.Case management in a heterogeneous congestive heart failure population. Archives of Internal Medicine 2003;163:809-17. [DOI] [PubMed] [Google Scholar]
Legrain 2011 {published data only}
- Bonnet-Zamponi D, D'Arailh L, Konrat C, Delpierre S, Lieberherr D, Lemaire A, et al, Optimzation of Medication in AGEd study group.Drug-related readmissions to medical units of older adults discharged from acute geriatric units: results of the optimization of medication in AGEd multicenter randomized controlled trial. Journal of the American Geriatrics Society 2013;61(1):113-21. [DOI] [PubMed] [Google Scholar]
- Legrain S, Tubach F, Bonnet-Zamponi D, Lemaire A, Aquino J, Paillaud E, et al.A new multimodal geriatric discharge planning intervention to prevent emergency visits and rehospitalizations of older adults: the optimization of medication in AGEd multicentre randomised controlled trial. Journal of the American Geriatric Society 2011;59(11):2017-28. [DOI] [PubMed] [Google Scholar]
Lin 2009 {published data only}
- Lin PC, Wang CH, Chen CS, Liao LP, Kao SF, Wu HF.To evaluate the effectiveness of a discharge planning programme for hip fracture patients. Journal of Clinical Nursing 2009;18(11):1632-9. [DOI] [PubMed] [Google Scholar]
Lindpaintner 2013 {published data only}
- Lindpaintner LS, Gasser JT, Schramm MS, Cina-Tschumi B, Müller B, Beer JH.Discharge intervention pilot improves satisfaction for patients and professionals. European Journal of Internal Medicine 2013;24(8):756-62. [DOI] [PubMed] [Google Scholar]
Lisby 2019 {published data only}
- Lisby M, Klingenberg M, Ahrensberg JM, Hoeyem PH, Kirkegaard H.Clinical impact of a comprehensive nurse-led discharge intervention on patients being discharged home from an acute medical unit: randomised controlled trial. International Journal of Nursing Studies 2019;100:103411. [DOI: 10.1016/j.ijnurstu.2019.103411] [DOI] [PubMed] [Google Scholar]
- NCT02295319.The impact of individual-based discharges from acute admission units to home. clinicaltrials.gov/ct2/show/NCT02295319 (accessed 2 June 2015).
Moher 1992 {published data only}
- Moher D, Weinberg A, Hanlon R, Runnalls K.Effects of a medical team coordinator on length of hospital stay. Canadian Medical Association Journal 1992;146(4):511-5. [PMC free article] [PubMed] [Google Scholar]
Naji 1999 {published data only}
- Naji SA, Howie FL, Cameron IM, Walker SA, Andrew J, Eagles JM.Discharging psychiatric in-patients back to primary care: a pragmatic randomized controlled trial of a novel discharge protocol. Primary Care Psychiatry 1999;5(3):109-15. [Google Scholar]
Naughton 1994 {published data only}
- Naughton B, Moran M, Feinglass J, Falconer J.Reducing hospital costs for the geriatric patient admitted from the emergency department: a randomised trial. Journal of the American Geriatrics Society 1994;42(10):1045-9. [DOI] [PubMed] [Google Scholar]
Naylor 1994 {published data only}
- Naylor M, Brooten D, Jones R, Lavizzo-Mourey R, Mezey M, Pauly M.Comprehensive discharge planning for the hospitalized elderly. A randomized clinical trial. Annals of Internal Medicine 1994;120(12):999-1006. [DOI] [PubMed] [Google Scholar]
Nazareth 2001 {published data only}
- Nazareth I, Burton A, Shulman S, Smith P, Haines A, Timberal H.A pharmacy discharge plan for hospitalized elderly patients - a randomized controlled trial. Age and Ageing 2001;30(1):33-40. [DOI] [PubMed] [Google Scholar]
Nguyen 2018 {published data only}
- Nguyen T, Nguyen PT, Tran HT, Nguyen NV, Nguyen HQ, Ha BN, et al.Pharmacist-led intervention to enhance medication adherence in patients with acute coronary syndrome in Vietnam: a randomized controlled trial. Pharmacoepidemiology and Drug Safety 2017;26:431-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Nguyen TH, Nguyen PT, Tran HT, Nguyen NV, Nguyen HQ, et al.Pharmacist-led intervention to enhance medication adherence in patients with acute coronary syndrome in Vietnam: a randomized controlled trial. Frontiers in Pharmacology 2018;9:656. [DOI: 10.3389/fphar.2018.00656] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parfrey 1994 {published data only}
- Parfrey PS, Gardner E, Vavasour H, Harnett JD, McManamon C, McDonald J, et al.The feasibility and efficacy of early discharge planning initiated by the admitting department in two acute care hospitals. Clinical and Investigative Medicine 1994;17(2):88-96. [PubMed] [Google Scholar]
Preen 2005 {published data only}
- Preen DB, Preen DB, Bailey BES, Wright A, Kendall P, Phillips M, et al.Effects of a multidisciplinary, post discharge continuance of care intervention on quality of life, discharge satisfaction, and hospital length of stay: a randomized controlled trial. International Journal for Quality in Health Care 2005;17(1):43-51. [DOI] [PubMed] [Google Scholar]
Rich 1993 {published data only}
- Rich MW, Vinson JM, Sperry JC, Shah AS, Spinner LR, Chung M, et al.Prevention of readmission in elderly patients with congestive heart failure: results of a prospective randomised pilot study. Journal of General Internal Medicine 1993;8(11):585-90. [DOI] [PubMed] [Google Scholar]
Rich 1995 {published data only}
- Rich MW, Beckham V, Wittenberg C, Leven C, Freedland KE, Carney RM.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. New England Journal of Medicine 1995;333(18):1190-5. [DOI] [PubMed] [Google Scholar]
Shaw 2000 {published data only}
- Shaw H, Mackie CA, Sharkie I.Evaluation of effect of pharmacy discharge planning on medication problems experienced by discharged acute admission mental health patients. International Journal of Pharmacy Practice 2000;8(2):144-53. [Google Scholar]
Sulch 2000 {published data only}
- Sulch D, Perez I, Melbourn A, Kalra L.Randomized controlled trial of integrated (managed) care pathway for stroke rehabilitation. Stroke 2000;31(8):1929-34. [DOI] [PubMed] [Google Scholar]
Weinberger 1996 {published data only}
- Weinberger M, Oddone EZ, Henderson WG.Does increased access to primary care reduce hospital admissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmissions. New England Journal of Medicine 1996;334(22):1441-7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abadi 2017 {published data only}
- Abadi TSH, Ghiyasvandian S, Moghadam MZ, Kazemnejad A.Self-determination theory application in the discharge of patients with transient ischemic attack. Advances in Bioscience & Clinical Medicine 2017;5:31. [DOI: 10.7575/aiac.abcmed.ca1.31] [DOI] [Google Scholar]
Applegate 1990 {published data only}
- Applegate WB, Miller ST, Graney MJ, Elam JT, Akins DE.A randomized controlled trial of a geriatric assessment unit in a community rehabilitation hospital. New England Journal of Medicine 1990;322(22):1572-8. [DOI] [PubMed] [Google Scholar]
Borenstein 2016 {published data only}
- Borenstein JE, Aronow HU, Bolton LB, Dimalanta MI, Chan E, Palmer K, et al.Identification and team-based interprofessional management of hospitalized vulnerable older adults. Nursing Outlook 2016;64(2):137-45. [DOI] [PubMed] [Google Scholar]
Brooten 1987 {published data only}
- Brooten D, Kumar S, Brown LP, Butts P, Finkler SA, Bakewell-Sachs S, et al.A randomized clinical trial of early hospital discharge and home follow-up of very-low-birth-weight infants. NLN Publications 1987;21-2194:95-106. [PubMed] [Google Scholar]
Brooten 1994 {published data only}
- Brooten D, Roncoli M, Finkler S, Arnold L, Cohen A, Mennuti M.A randomized trial of early hospital discharge and home follow-up of women having cesarean birth. Obstetrics and Gynecology 1994;84(5):832-8. [PMC free article] [PubMed] [Google Scholar]
Casiro 1993 {published data only}
- Casiro OG, McKenzie ME, McFadyen L, Shapiro C, Seshia MM, MacDonald N, et al.Earlier discharge with community-based intervention for low birth weight infants: a randomized trial. Pediatrics 1993;92(1):128-34. [PubMed] [Google Scholar]
Chen 2017 {published data only}
- Chen L, Chen Y, Chen X, Shen X, Wang Q, Sun C.Longitudinal effectiveness of a patient-centered self-management empowerment intervention during transition care on stroke survivors. Worldviews on Evidence-Based Nursing 2018;15(3):197-205. [DOI] [PubMed] [Google Scholar]
- Chen L.Effectiveness of a patient-centered self-management empowerment intervention during transition care on stroke survivors. repository.lib.cuhk.edu.hk/en/item/cuhk-1839362?solr_nav%5Bid%5D=98955229c6430ff80b50&solr_nav%5Bpage%5D=0&solr_nav%5Boffset%5D=2 (accessed 30 September 2020).
Choong 2000 {published data only}
- Choong PF, Langford AK, Dowsey MM, Santamaria NM.Clinical pathway for fractured neck of femur: a prospective controlled study. Medical Journal of Australia 2000;172(9):423-6. [DOI] [PubMed] [Google Scholar]
Clemson 2016 {published data only}
- Clemson L, Lannin NA, Wales K, Salkeld G, Rubenstein L, Gitlin L, et al.Occupational therapy predischarge home visits in acute hospital care: a randomized trial. JAGS 2016;64(10):2019-26. [DOI: 10.1111/jgs.14287] [DOI] [PubMed] [Google Scholar]
Diplock 2017 {published data only}
- Diplock G, Ward J, Stewart S, Scuffham P, Stewart P, Reeve, C, et al.The Alice Springs Hospital Readmission Prevention Project (ASHRAPP): a randomised control trial. BMC Health Services Research 2017;17(1):153. [DOI: 10.1186/s12913-017-2077-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Drummond 2012 {published data only}
- Drummond A, Whitehead P, Fellows K, Sprigg N, Sampson C, Edwards C.Pre-discharge occupational therapy home visits for patients with a stroke: results of a feasibility randomised controlled trial (RCT). International Journal of Stroke 2012;7:6. [DOI] [PubMed] [Google Scholar]
- Sprigg N, Drummond AE, Whitehead PJ, Fellows KR, Sampson CJ, Edwards C, et al.Results of the home visit after stroke (HOVIS) feasibility randomised controlled trial (RCT). Cerebrovascular Diseases 2013;35:784. [DOI] [PubMed] [Google Scholar]
Englander 2014 {published data only}
- Englander H, Michaels L, Chan B, Kansagara D.The care transitions innovation (C-TraIn) for socioeconomically disadvantaged adults: results of a cluster randomized controlled trial. Journal of General Internal Medicine 2014;29(11):460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Germain 1995 {published data only}
- Germain M, Knoeffel F, Wieland D, Rubenstein LZ.A geriatric assessment and intervention team for hospital inpatients awaiting transfer to a geriatric unit: a randomized trial. Aging (Milan, Italy) 1995;7(1):55-60. [DOI] [PubMed] [Google Scholar]
González‐Guerrero 2014 {published data only}
- González-Guerreroa JL, Alonso-Fernándeza T, García-Mayolín N, Gusi N, Ribera-Casado JM.Effectiveness of a follow-up program for elderly heart failure patients after hospital discharge. A randomized controlled trial. European Geriatric Medicine 2014;5(4):252-7. [DOI] [PubMed] [Google Scholar]
Haggmark 1997 {published data only}
- Häggmark C, Nilsson B.Effects of an intervention programme for improved discharge planning. Nordic Journal of Nursing Research 1997;17(2):4-8. [Google Scholar]
Hegelund 2019 {published data only}
- Hegelund A, Andersen IC, Andersen MN, Bodtger U.The impact of a personalised action plan delivered at discharge to patients with COPD on readmissions: a pilot study. Scandinavian Journal of Caring Sciences 2019. [DOI: 10.1111/scs.12798] [DOI] [PMC free article] [PubMed]
Hickey 2000 {published data only}
- Hickey ML, Cook FE, Rossi LR, Connor J, Dutkiewicz C, McCabe Hassan S, et al.Effect of case managers with a general medical patient population. Journal of Evaluation in Clinical Practice 2000;6(1):23-9. [DOI] [PubMed] [Google Scholar]
IRCT2016072119141N2 {published data only}
- IRCT2016072119141N2.The effect of family caregiver-oriented discharge planning program on nutritional status, laboratory nutritional parameters and quality of life in patient suffering from hip fractures. en.irct.ir/trial/17198 (first received 30 September 2016).
Jenkins 1996 {published data only}
- Jenkins HM, Blank V, Miller K, Turner J, Stanwick RS.A randomized single-blind evaluation of a discharge teaching book for pediatric patients with burns. Journal of Burn Care & Rehabilitation 1996;17(1):49-61. [PubMed] [Google Scholar]
JPRN‐UMIN000029404 {published data only}
- JPRN-UMIN000029404.A single-center, prospective, non-blinded, randomized, 12-month, parallel-group, superiority study to compare the efficacy of pharmacist intervention versus usual care for older patients hospitalized in the orthopedic ward. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000033602 (first received 3 October 2017).
- Komagamine J, Sugawara K, Kaminaga M, Tatsumi S.Study protocol for a single-centre, prospective, non-blinded, randomised, 12-month, parallel-group superiority study to compare the efficacy of pharmacist intervention versus usual care for elderly patients hospitalised in orthopaedic wards. BMJ Open 2018;8:e021924. [DOI: 10.1136/bmjopen-2018-021924] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karppi 1995 {published data only}
- Karppi P, Tilvis R.Effectiveness of a Finnish geriatric inpatient assessment. Two-year follow up of a randomized clinical trial on community-dwelling patients. Scandinavian Journal of Primary Health Care 1995;13(2):93-8. [DOI] [PubMed] [Google Scholar]
Kempen 2020 {published data only}
- Kempen T, Bertilsson M, Hadziosmanovic N, Lindner K-J, Melhus H, Nielsen E, et al.Effectiveness of hospital-based comprehensive medication reviews including post-discharge follow-up on older patients' healthcare utilisation (the MedBridge trial): pragmatic cluster randomized crossover trial. Journal of the American College of Clinical Pharmacy 2020;3(8):1583‐2020. [Google Scholar]
Kleinpell 2004 {published data only}
- Kleimpell RM.Randomized trial of an intensive care unit-based early discharge planning intervention for critically ill elderly patients. American Journal of Critical Care 2004;13(4):335-45. [PubMed] [Google Scholar]
Lang 2017 {published data only}
- Lang DS, Lim BK.Nurse-led discharge care protocol: a randomised control trial. Singapore Nursing Journal 2017;44(3):2-6. [Google Scholar]
Linden 2014 {published data only}
- Linden A, Butterworth S.A comprehensive hospital-based intervention to reduce readmissions for chronically ill patients: a randomized controlled trial. American Journal of Managed Care 2014;20(10):783-92. [PubMed] [Google Scholar]
Lindhardt 2019 {published data only}
- Lindhardt T, Loevgreen SM, Bang B, Bigum C, Klausen TW.A targeted assessment and intervention at the time of discharge reduced the risk of readmissions for short-term hospitalized older patients: a randomized controlled study. Clinical Rehabilitation 2019;33(9):1431-44. [DOI: 10.1177/0269215519845032] [DOI] [PubMed] [Google Scholar]
- NCT01843907.Patient participation in prevention of loss of functions. clinicaltrials.gov/ct2/show/NCT01843907 (first received 1 May 2013).
Lisby 2018 {published data only}
- Lisby M, Bonnerup DK, Brock B, Gregersen PA, Jensen J, Larsen ML, et al.Medication review and patient outcomes in an orthopedic department: a randomized controlled study. Journal of Patient Safety 2018;14(2):74-81. [DOI] [PubMed] [Google Scholar]
Loffler 2014 {published data only}
- Löffler C, Drewelow E, Paschka SD, Frankenstein M, Eger L, Jatsch L, et al.Optimizing polypharmacy among elderly hospital patients with chronic diseases—study protocol of the cluster randomized controlled POLITE-RCT trial. Implementation Science 2014;9:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopes Oscalices 2019 {published data only}
- Lopes Oscalices MI, Okuno MF, Lopes MC, Campanharo CR, Batists RE.Discharge guidance and telephone follow-up in the therapeutic adherence of heart failure: randomized clinical trial. Resvista Latino-Americana de Enfermagem 2019;27:e3159. [DOI: 10.1590/1518-8345.2484.3159] [DOI] [PMC free article] [PubMed] [Google Scholar]
Luo 2019 {published data only}
- Luo X-H, Deng L-C, Zhang Y-F, Huang X-R, Chen D-F.Application of discharge planning in rectal cancer patients with a stoma. World Chinese Journal of Digestology 2019;27(7):435-41. [Google Scholar]
Martin 1994 {published data only}
- Martin F, Oyewole A, Moloney A.A randomized controlled trial of a high support hospital discharge team for elderly people. Age and Ageing 1994;23(3):228-34. [DOI] [PubMed] [Google Scholar]
Martin‐Sanchez 2019 {published data only}
- Martin-Sanchez FJ, Llopis Garcia G, Llorens P, Jacob J, Herrero P, Gil V, et al.Planning to reduce 30-day adverse events after discharge of frail elderly patients with acute heart failure: design and rationale for the DEED FRAIL-AHF trial. Emergencias 2019;31(1):27-35. [PubMed] [Google Scholar]
- NCT03696875.Discharge planning in emergency department for frail older with AHF. clinicaltrials.gov/ct2/show/NCT03696875 (first received 5 October 2018).
Marusic 2013 {published data only}
- Marusic S, Gojo-Tomic N, Erdeljic V, Bacic-Vrca V, Franic M, Kirin M, et al.The effect of pharmacotherapeutic counseling on readmissions and emergency department visits. International Journal of Clinical Pharmacy 2013;35(1):37-44. [DOI] [PubMed] [Google Scholar]
McGrory 1994 {published data only}
- McGrory A, Assmann S.A study investigating primary nursing, discharge teaching, and patient satisfaction of ambulatory cataract patients. Insight 1994;19(2):8-13, 29. [PubMed] [Google Scholar]
McInnes 1999 {published data only}
- McInnes E, Mira M, Atkin N, Kennedy P, Cullen J.Can GP input into discharge planning result in better outcomes for the frail aged: results from a randomized controlled trial. Family Practice 1999;16(3):289-93. [DOI] [PubMed] [Google Scholar]
Naylor 1999 {published data only}
- Naylor MD, Brooten D, Campbell R, Jacobsen BS, Mezey MD, Pauly MV, et al.Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized controlled trial. JAMA 1999;281(7):613-20. [DOI] [PubMed] [Google Scholar]
Naylor 2004 {published data only}
- Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Sanford Schwartz J.Transitional care of older adults hospitalized with heart failure: a randomised controlled trial. Journal of the American Geriatrics Society 2004;52(5):675-84. [DOI] [PubMed] [Google Scholar]
NCT02112227 {published data only}
- NCT02112227.Patient-centered Care Transitions in Heart Failure (PACT-HF). clinicaltrials.gov/ct2/show/NCT02112227 (accessed 2 June 2015).
- Van Spall HG, Lee SF, Xie F, Ko DT, Thabane L, Ibrahim Q, et al.Knowledge to action: rationale and design of the Patient-Centered Care Transitions in Heart Failure (PACT-HF) stepped wedge cluster randomized trial. American Heart Journal 2018;199:75-82. [DOI: 10.1016/j.ahj.2017.12.013] [DOI] [PubMed] [Google Scholar]
- Van Spall HG, Lee SF, Xie F, Oz UE, Perez R, Mitoff PR, et al.Effect of Patient-Centered Transitional Care Services on Clinical Outcomes in Patients Hospitalized for Heart Failure: The PACT-HF randomized clinica trial. JAMA 2019;321(8):753-61. [DOI: 10.1001/jama.2019.0710] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02351648 {published data only}
- NCT02351648.A randomised control trial of a transitional care model in Singapore General Hospital. clinicaltrials.gov/ct2/show/NCT02351648 (accessed 2 June 2015).
NCT03258632 {published data only}
- NCT03258632.Improving outcomes among medical/surgical inpatients with alcohol use disorders. clinicaltrials.gov/ct2/show/NCT03258632 (first received 23 August 2017).
Nickerson 2005 {published data only}
- Nickerson A, McKinnon NJ, Roberst N, Saulnier L.Drug therapy problems, inconsistencies and omissions identified during a medication reconciliation and seamless care service. Healthcare Quarterly 2005;8(Spec No):65-72. [DOI] [PubMed] [Google Scholar]
Pourrat 2017 {published data only}
- Pourrat X, Roux C, Bouzige B, Garnier V, Develay A, Fraysse M, et al.Impact of drug reconciliation at discharge and communication between hospital and community pharmacists on drug-related problems: a randomized controlled trial. International Journal of Clinical Pharmacy 2017;39(1):212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Puschner 2008 {published data only}
- Puschner B, Baumgartner I, Loos S, Völker KA, Ramacher M, Sohla K, et al.Cost-effectiveness of needs-oriented discharge planning in high utilizers of mental health care [Kosteneffektivität bedarfsorientierter Entlassungsplanungbei Menschen mit hoher Inanspruchnahmepsychiatrischer Versorgung]. Psychiatrische Praxis 2012;39:381-7. [DOI: 10.1055/s-0032-1327188] [DOI] [PubMed] [Google Scholar]
- Puschner B, Steffen S, Gaebel W, Freyberger H, Klein HE, Steinert T, et al.Needs-oriented discharge planning and monitoring for high utilisers of psychiatric services (NODPAM): design and methods. BMC Health Services Research 2008;8:152. [DOI: 10.1186/1472-6963-8-152] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puschner B, Steffen S, Völker KW, Spitzer C, Gaebel W, Janssen B, et al.Needs-oriented discharge planning for high utilisers of psychiatric services: multicentre randomised controlled trial. Epidemiology and Psychiatric Sciences 2011;20:181-92. [DOI: 10.1017/S2045796011000278] [DOI] [PubMed] [Google Scholar]
Ravn‐Nielsen 2018 {published data only}
- Ravn-Nielsen LV, Duckert, M-L, Lund ML, Henriksen JP, Nielsen ML, Eriksen CS, et al.The impact of an in-hospital multifaceted clinical pharmacist intervention on the risk of readmission: a randomized clinical trial. Pharmacoepidemiology and Drug Safety 2018;27:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rich 1993b {published data only}
- Rich MW, Vinson JM, Sperry JC, Shah AS, Spinner LR, Chung MK, et al.Prevention of readmission in elderly patients with congestive heart failure: results of a prospective, randomized pilot study. Journal of General Internal Medicine 1993;8(11):585-90. [DOI] [PubMed] [Google Scholar]
Rich 1995b {published data only}
- Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. New England Journal of Medicine 1995;333(18):1190-5. [DOI] [PubMed] [Google Scholar]
Saleh 2012 {published data only}
- Saleh SS, Freire C, Morris-Dickinson G, Shannon T.An effectiveness and cost-benefit analysis of a hospital-based discharge transition program for elderly Medicare recipients. Journal of the American Geriatrics Society 2012;60(6):1051-6. [DOI] [PubMed] [Google Scholar]
Salmani 2018 {published data only}
- Salmani S, Imanipour M, Nikbakht Nasrabadi A.Implementation of a discharge planning to improve quality of life in breast cancer patients: a quasi-experimental study. Archives of Breast Cancer 2018;5(4):163-7. [DOI: 10.32768/abc.201854163-167] [DOI] [Google Scholar]
Schnipper 2021 {published data only}
- Schnipper JL, Samal L, Nolido N, Yoon C, Dalal AK, Magny-Normilus C, et al.The effects of a multifaceted intervention to improve care transitions within an accountable care rrganization:results of a stepped wWedge cluster-randomized trial. Journal of Hospital Medicine 2021;16(1):15-22. [DOI: doi: 10.12788/jhm.3513. PMID: 33357325; PMCID: PMC7768916.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2013 {published data only}
- Shah M, Norwood CA, Farias S, Ibrahim S, Chong PH, Fogelfeld L.Diabetes transitional care from inpatient to outpatient setting: pharmacist discharge counseling. Journal of Pharmacy Practice 2013;26(2):120-4. [DOI] [PubMed] [Google Scholar]
Sharif 2014 {published data only}
- Sharif F, Moshkelgosha F, Molazem Z, Najafi Kalyani M, Vossughi M.The effects of discharge plan on stress, anxiety and depression in patients undergoing percutaneous transluminal coronary angioplasty: a randomized controlled trial. International Journal of Community Based Nursing & Midwifery 2014;2(2):60-8. [PMC free article] [PubMed] [Google Scholar]
Shyu 2010 {published data only}
- Shyu YI, Liang J, Wu CC, Su JY, Cheng HS, Chou SW, et al.Two-year effects of interdisciplinary intervention for hip fracture in older Taiwanese. Journal of the American Geriatrics Society 2010;58(6):1081-9. [DOI] [PubMed] [Google Scholar]
Townsend 1988 {published data only}
- Townsend J, Piper M, Frank AO, Dyer S, North WR, Meade TW.Reduction in hospital readmission stay of elderly patients by a community based hospital discharge scheme: a randomized controlled trial. BMJ 1988;297(6647):544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tseng 2012 {published data only}
- Tseng MY, Shyu YI, Liang J.Functional recovery of older hip-fracture patients after interdisciplinary intervention follows three distinct trajectories. Gerontologist 2012;52(6):833-42. [DOI] [PubMed] [Google Scholar]
Van Hollebeke 2016 {published data only}
- Van Hollebeke M, Talavera-Pons S, Mulliez A, Sautou V, Bommelae, G, Abergel A, et al.Impact of medication reconciliation at discharge on continuity of patient care in France. International Journal of Clinical Pharmacy 2016;38(5):1149-56. [DOI] [PubMed] [Google Scholar]
Victor 1988 {published data only}
- Victor CR, Vetter NJ.Rearranging the deckchairs on the Titanic: failure of an augmented home help scheme after discharge to reduce the length of stay in hospital. Archives of Gerontology and Geriatrics 1988;7(1):83-91. [DOI] [PubMed] [Google Scholar]
Voirol 2004 {published data only}
- Voirol P, Kayser SR, Chang CY, Chang QL, Youmans SL.Impact of pharmacists' interventions on the pediatric discharge medication process. Annals of Pharmacotherapy 2004;38(10):1597-602. [DOI] [PubMed] [Google Scholar]
Xu 2019 {published data only}
- Xu H, Zou J, Ye X, Han J, Gao L, Luo S, et al.Impacts of clinical pharmacist intervention on the secondary prevention of coronary heart disease: a randomized controlled clinical study. Frontiers in Pharmacology 2019;10:1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yeung 2012 {published data only}
- Yeung, SM.The Effects of a Transitional Care Programme Using Holistic Care Interventions for Chinese Stroke Survivors and Their Care Providers: a Randomized Controlled Trial. Hong Kong: Hong Kong Polytechnic University, 2012. [Google Scholar]
References to ongoing studies
DRKS00015996 {published data only}
- DRKS00015996.Vun nix kütt nix - patient, geriatrician and general practitioner as a multiprofessional team for intersectoral discharge management. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00015996 (first received 26 February 2019).
Ergan 2018 {published data only}
- Ergan B, Goktalay T, Ergun P, Yilmaz D, Ocakli B, Gurgun A, et al.A multicenter randomized trial for the effectiveness of structured discharge and follow up protocol on readmission rate in COPD patients receiving LTOT/NIV: one year interim analysis. European Respiratory Journal 2018;52(Suppl. 62):OA5410. [DOI: 10.1183/13993003.congress-2018.OA5410] [DOI] [Google Scholar]
Grischott 2018 {published data only}
- Grischott T, Zechmann S, Rachamin Y, Markun S, Chmiel C, Senn O, et al.Improving inappropriate medication and information transfer at hospital discharge: study protocol for a cluster RCT. Implementation Science 2018;13:155. [DOI: 10.1186/s13012-018-0839-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02388711 {published data only}
- NCT02388711.A trial of the C-TraC intervention for dementia patients. clinicaltrials.gov/ct2/show/NCT02388711 (accessed 2 June 2015).
NCT02421133 {published data only}
- NCT02421133.Impact of a transitional care program on 30-day hospital readmissions for elderly patients discharged from a short stay geriatric ward (PROUST). clinicaltrials.gov/ct2/show/NCT02421133 (accessed 2 June 2015).
- Occelli P, Touzet S, Rabilloud M, Ganne C, Poupon Bourdy S, Galamand B, et al.Impact of a transition nurse program on the prevention of thirty-day hospital readmissions of elderly patients discharged from short-stay units: study protocol of the PROUST stepped-wedge cluster randomised trial. BMC Geriatrics 2016;16:57. [DOI: 10.1186/s12877-016-0233-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03358771 {published data only}
- NCT03358771.COPD discharge bundle delivered alone or enhanced through a care coordinator (PRIHS). clinicaltrials.gov/ct2/show/NCT03358771 (first received 2 December 2017).
NCT03496896 {published data only}
- NCT03496896.Transition cAre inteRvention tarGeted to High-risk patiEnts To Reduce rEADmission (TARGET-READ). clinicaltrials.gov/ct2/show/NCT03496896 (first received April 2018).
NCT04154917 {published data only}
- NCT04154917.Effectiveness of a comprehensive patient-centered hospital discharge planning intervention for frail older adults (HOME). clinicaltrials.gov/ct2/show/NCT04154917 (first received 7 November 2019).
Additional references
Aronson 2017
- Aronson J.Medication reconciliation. BMJ 2017;356:i5336 doi:10.1136/bmj.i5336. [DOI] [PubMed] [Google Scholar]
Bibbins‐Domingo 2019
- Bibbins-Domingo K.Integrating social careiInto the delivery of health care. JAMA 2019;322(18):1763-4. [DOI] [PubMed] [Google Scholar]
Burgess 2014
- Burgess JF, Hockenberry JM.Can all cause readmission policy improve quality or lower expenditures? A historical perspective on current initiatives. Health Economics, Policy and Law 2014;9(2):193-213. [DOI] [PubMed] [Google Scholar]
Campbell 2020
- Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al.Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020;368:l6890 doi:10.1136/bmj.l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Care Quality Commission 2020
- Care Quality Commission.Medicines reconciliation (how to check you have the right medicines). Care Quality Commission (cqc.org.uk) July 2020.
ClinicalTrials.gov
- ClinicalTrialsgov.ClinicalTrials.gov. clinicaltrials.gov (accessed 2 April 2020).
Cochran 1954
- Cochran WG.The combination of estimates from different experiments. Biometrics 1954;10:101-29. [Google Scholar]
Dept of Health 2020
- Department of Health & Social Care.Hospital discharge service: policy and operating model. assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/912199/Hospital_Discharge_Policy_1.pdf (accessed 28 September 2020).
DHHS 2019
- Department of Health and Human Services.Burden Reduction and Discharge Planning Final Rules Guidance and Process. www.hhs.gov/guidance/sites/default/files/hhs-guidance-documents/QSO20-07%2001%20Burden%20Reduction-Discharge%20Planning%20SOM%20Package121919.pdf (accessed 28 September 2020).
Ellis 2017
- Ellis G, Gardner M, Tsiachristas A, Langhorne P, Burke O, Harwood RH, et al.Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No: CD006211. [DOI: 10.1002/14651858.CD006211.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2015
- Effective Practice and Organisation of Care (EPOC).Data extraction and management. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services; 2013. Available at: http://epoc.cochrane.org/epoc-specific-resources-review-authors.
EPOC 2017
- Cochrane Effective Practice and Organisation of Care (EPOC).EPOC Resources for review authors: Synthesising results when it does not make sense to do a meta-analysis. epoc.cochrane.org/resources/epoc-resources-review-authors 2017;Accessed 23/09/21.
Finkelstein 2020
- Finkelstein A, Zhou A, Taubman S, Doyle J.Health care hotspotting - a randomized, controlled trial. New England Journal of Medicine 2020;9(382):152-62. [DOI: 10.156/NEJMsa1906848. PMID: 31914242; PMCID: PMC7046127] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2021 [Computer program]
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software].Available from www.gradepro.org: McMaster University and Evidence Prime, 2021.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist G, Kunz R, Falck-YtterY, Alonso-Coello P, et al for the GRADEWorking Group.Rating quality of evidence and strength of recommendations GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Health Direct 2020
- Health Direct Australia.Hospital discharge planning. www.healthdirect.gov.au/hospital-discharge-planning (accessed 28 September 2020).
Health Qual Ontario 2013
- Health Quality Ontario.Adopting a Common Approach to Transitional Care Planning: Helping Health Links Improve Transitions and Coordination of Care. Health Quality Ontario, 2013. [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG.Measuring inconsistency in meta-analysis. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT Green S (editors).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org, 2011. [Google Scholar]
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors).Cochrane Handbook for Systematic Reviews of Interventions.. 2nd Edition. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
Landeiro 2019
- Landeiro F, Roberts K, Gray AM, Leal J.Delayed hospital discharges of older patients: a systematic review on prevalence and costs. Gerontologist 2019;59(2):e86–e97, https://doi.org/10.1093/geront/gnx028. [DOI] [PubMed] [Google Scholar]
Langhorne 2020
- Langhorne P, Ramachandra S.Organised inpatient (stroke unit) care for stroke: network meta‐analysis. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD000197. [DOI: 10.1002/14651858.CD000197.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al.Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, et al (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021) 2021:Available from: www.training.cochrane.org/handbook.
Leppin 2014
- Leppin AL, Gionfriddo MR, Kessler M, Brito JP, Mair FS, Gallacher K, et al.Preventing 30-day hospital readmissions: A systematic review and meta-analysis of randomized trials. JAMA Internal Medicine 2014;174(7):1095-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mabire 2016
- Mabire C, Dwyer A, Garnier A, Pellet J.Effectiveness of nursing discharge planning interventions on health-related outcomes in discharged elderly inpatients: a systematic review. JBI Database of Systematic Reviews and Implementation Reports 2016;14(9):217-60. [DOI: 10.11124/JBISRIR-2016-003085 ] [DOI] [PubMed] [Google Scholar]
Mäkelä 2020
- Mäkelä P, Stott DJ, Godfrey M, Elli, G, Schiff R, Shepperd S.The work of older people and their informal caregivers in managing an acute health event in a hospital at home or hospital inpatient setting. Age and Ageing 2020;49:856-64. [DOI: 10.1093/ageing/afaa085] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marks 1994
- Marks L.Seamless Care or Patchwork Quilt? Discharging Patients from Acute Hospital Care. London: Kings Fund, 1994. [Google Scholar]
NHS 2020
- Statistical press notice: monthly delayed transfers of care data, England. February 2020. www.england.nhs.uk/statistics/wp-content/uploads/sites/2/2020/04/February-20-DTOC-SPN-JQU3J.pdf (first accessed 21 January 2021).
NHS Long Term Plan 2019
- NHS England.NHS Long Term Plan. NHS Long Term Plan » Online version of the NHS Long Term Plan August 2019;version 1.2.
Pardessus 2002
- Pardessus V, Puisieux F, Di Pompeo C, Gaudefroy C, Thevenon A, Dewailly P.Benefits of home visits for falls and autonomy in the elderly: a randomized trial stud. American Journal of Physical Medicine & Rehabilitation 2002;81(4):247-52. [DOI] [PubMed] [Google Scholar]
Parker 2002
- Parker SG, Peet SM, McPherson A, Cannaby AM, Abrams K, Baker R, et al.A systematic review of discharge arrangements for older people. Health Technology Assessment 2002;6(4):1-183. [DOI] [PubMed] [Google Scholar]
Phillips 2004
- Phillips CO, Wright SM, Kern DE, Singa RM, Shepperd S, Rubin HR.Comprehensive discharge planning with post discharge support for older patients with congestive heart failure: a meta-analysis. JAMA 2004;291(11):358-67. [DOI] [PubMed] [Google Scholar]
RCP 2017
- Royal College of Physicians.Delivering the future hospital. www.rcplondon.ac.uk/projects/outputs/future-hospital-programme-delivering-future-hospital (accessed 28 September 2020).
Redmond 2016
- Redmond P, Grimes TC, McDonnell R, Boland F, Hughes C, Fahey T.Impact of medication reconciliation for improving transitions of care. Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No: CD010791. [DOI: 10.1002/14651858.CD010791.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager.Review Manager (RevMan), Version Version 5.4. The Cochrane Collaboration, 2020. Computer program.
Sezgin 2020
- Sezgin D, O'Caoimh R, Liew A, O'Donovan MR, Illario M, Salem MA, S, et al, all EU ADVANTAGE Joint Action Work Package 7 partners.The effectiveness of intermediate care including transitional care interventions on function, healthcare utilisation and costs: a scoping review. European Geriatric Medicine 2020;11(6):961-74. [DOI: 10.1007/s41999-020-00365-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shepperd 2009
- Shepperd S, Lewin S, Straus S, Clarke M, Eccles MP, Fitzpatrick R, et al.Can We Systematically Review Studies That Evaluate Complex Interventions? PLOS Medicine 2009;6(8):e1000086. https://doi.org/10.1371/journal.pmed.1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ubbink 2014
- Ubbink DT, Tump E, Koenders JA, Kleiterp S, Goslings JC, Brölmann FE.Which reasons do doctors, nurses, and patients have for hospital discharge? A mixed-methods study. PLOS One 2014;9(3):e91333. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2019
- WHO 2019.Medication safety in transitions of care. TransitionOfCare31Print (who.int) 2019; Accessed 21 March 2021.
References to other published versions of this review
Gonçalves‐Bradley 2016
- Gonçalves-Bradley DC, Lannin NA, Clemson LM, Cameron ID, Shepperd S.Discharge planning from hospital. Cochrane Database of Systematic Reviews 2016, Issue 1. Art. No: CD000313. [DOI: 10.1002/14651858.CD000313.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parkes 2000
- Parkes J, Shepperd S.Discharge planning from hospital to home. Cochrane Database of Systematic Reviews 2000, Issue 3. Art. No: CD000313. [DOI: 10.1002/14651858.CD000313] [DOI] [PubMed] [Google Scholar]
Shepperd 2004
- Shepperd S, Parkes J, McClaren J, Phillips C.Discharge planning from hospital to home. Cochrane Database of Systematic Reviews 2004, Issue 1:CD000313. Art. No: CD000313. [DOI: 10.1002/14651858.CD000313.pub2] [DOI] [PubMed] [Google Scholar]
Shepperd 2010
- Shepperd S, McClaran J, Phillips CO, Lannin NA, Clemson LM, McCluskey A, et al.Discharge planning from hospital to home. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD000313. [DOI: 10.1002/14651858.CD000313.pub3] [DOI] [PubMed] [Google Scholar]
Shepperd 2013
- Shepperd S, Lannin NA, Clemson LM, McCluskey A, Cameron ID, Barras SL.Discharge planning from hospital to home. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No: CD000313. [DOI: 10.1002/14651858.CD000313.pub4] [DOI] [PubMed] [Google Scholar]


