Figure 1.
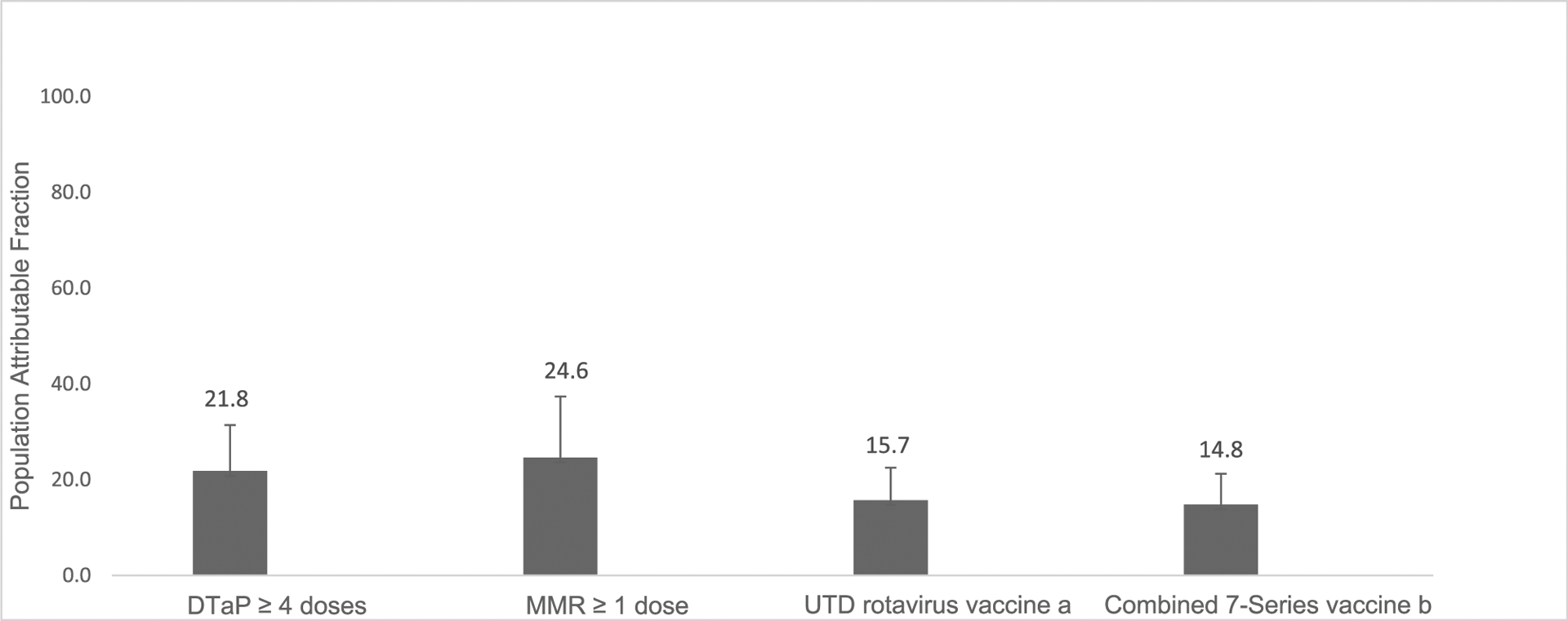
Population attributable fraction of vaccine hesitancy on undervaccination, April–June 2018 and 2019, NIS-Child.
aUTD defined as ≥3 rotavirus doses of any type or ≥2 Rotarix doses.
bThe combined 7-vaccine series (4:3:1:3*: 3:1:4) includes ≥4 doses of DTaP, ≥3 doses of IPV, ≥1 dose of MMR, the full series of Hib (≥3 or ≥4 doses, depending on product type), ≥3 doses of HepB, ≥1 dose of VAR, and ≥4 doses of PCV.
DTaP, diphtheria, tetanus toxoid, and acellular pertussis vaccine; HepB, hepatitis B vaccine; Hib, Haemophilus influenzae type b conjugate vaccine; IPV, inactivated polio vaccine; MMR, measles, mumps, and rubella vaccine; NIS, National Immunization Survey; PCV, pneumococcal conjugate vaccine; UTD, up-to-date; VAR, varicella vaccine.
