Fig. 2: Regulation of IRE1α and PERK signalling.
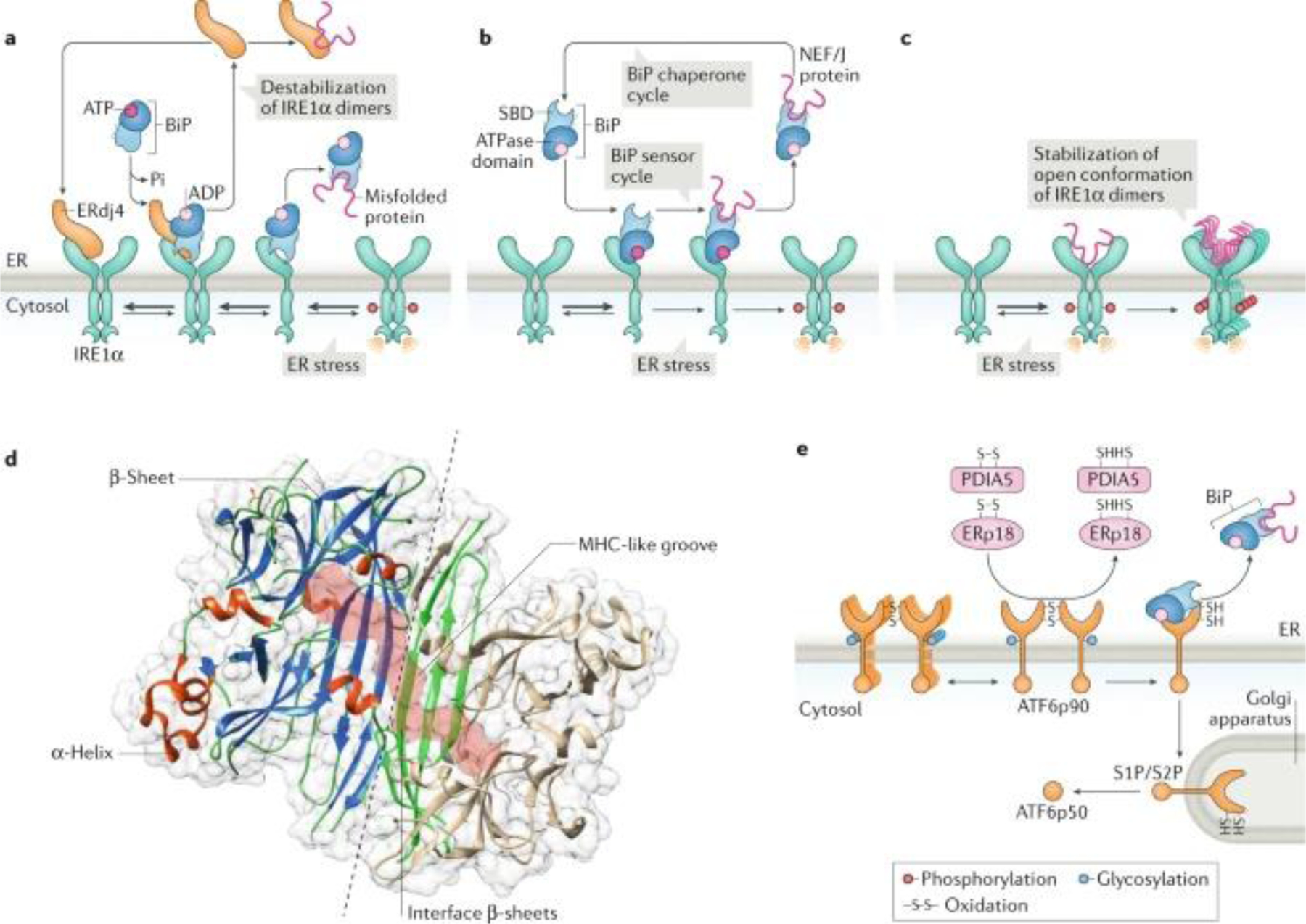
a | Indirect endoplasmic reticulum (ER) stress sensing model. In resting cells, the ER stress sensor IRE1α is maintained in an inactive state through its association with the ER chaperone BiP (also known as GRP78). On accumulation of unfolded proteins, BiP preferentially binds to unfolded protein peptides, thereby releasing the ER stress sensor to allow its spontaneous dimerization and activation. In this model, BiP has the capacity to destabilize IRE1α dimers and maintain the unfolded protein response transducer in an inactive state. In addition, the BiP co-chaperone ERdj4 is required for BiP binding to IRE1α and repression of IRE1α activation. b | Alternatively, BiP might bind misfolded proteins through the substrate-binding domain (SBD), which transduces a signal to the ATPase domain to release the repressive interaction over IRE1α and PERK. c | A direct recognition model proposes that unfolded proteins bind directly to the luminal domains of IRE1α, facilitating the assembly of highly ordered IRE1α clusters. This may orient the cytosolic region of the dimer to create a ribonuclease site and generate an mRNA docking region. d | The 3D structure of the ER luminal domain of yeast Ire1p is shown, depicting the dimeric interphase (dashed line) and the major histocompatibility complex (MHC) class-I like groove (pink surface), where misfolded peptides might bind. Protein Data Bank accession number 2BE1. e | ATF6 is regulated by its glycosylation and redox state, in addition to the binding of various disulfide isomerases, including PDIA5 and ERp18. ATF6p90, full-length AFT6; J protein, J-domain protien; NEF, nucleotide exchange factor; S1P, site-1 protease; S2P, site-2 protease.
