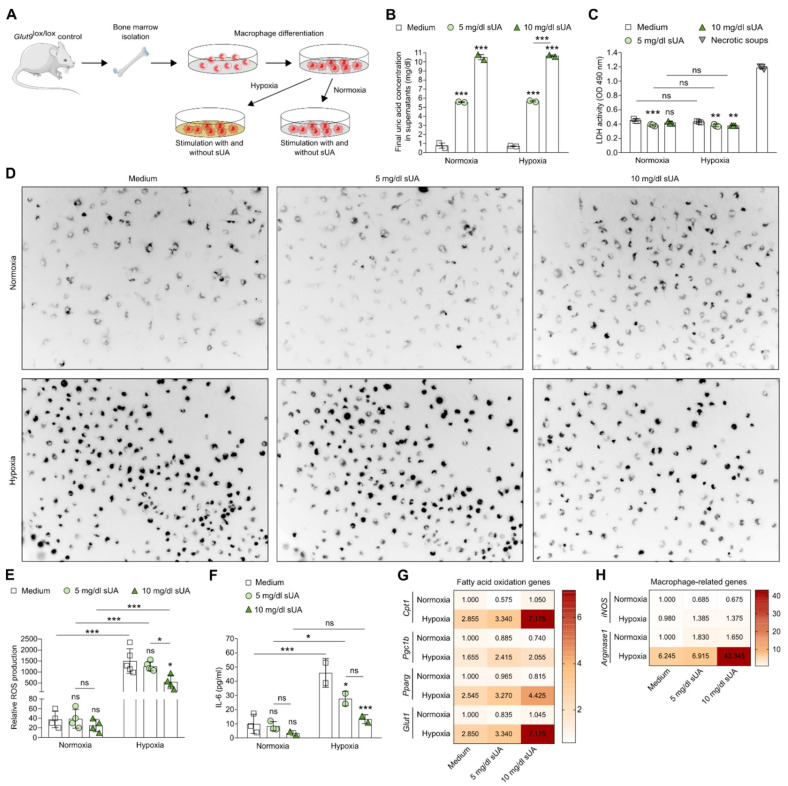
Figure 1.
Soluble uric acid inhibits pro-inflammatory functions and enhances the metabolic activity of macrophages under hypoxic conditions. (A) Schematic of experimental setup. Bone marrow-derived macrophages from Glut9lox/lox mice were primed with LPS for 24 h prior to sUA stimulation (medium; 5 mg/dL sUA; 10 mg/dL sUA). To mimic ischemic/reperfusion, cells were cultured under hypoxic (5% O2) and/or normoxic (21% O2) conditions for 24 h. (B) Final UA concentrations after adding 5 and 10 mg/dL sUA into the macrophage culture were measured by colorimetric assay. (C) Cytotoxicity (LDH activity) of macrophages was assessed by LDH assay (absorbance, OD 490 nm) (n = 3). DAMP-containing supernatants from necrotic tubular epithelial cells were used as positive control (necrotic soups). (D,E) Representative images (D) and quantification of ROS production (E) was detected with dihydrorhodamine 123 dye using a fluorescent microscope (n = 4–5). (F) Concentrations of interleukin (IL)-6 in culture supernatants were determined by ELISA (n = 2–3). (G) mRNA expression of genes associated with fatty acid oxidation Cpt1, Pgc1b, Pparg, and Glut1 was determined by RT-qPCR (n = 4–5). (H) mRNA expression of the M1-like pro-inflammatory gene iNos and the M2-like anti-inflammatory gene Arg-1 was determined by RT-qPCR (n = 4–5). Data are expressed as mean ± SEM. * p < 0.05; ** p < 0.01; *** p < 0.001; ns, not significant; two-way ANOVA with Tukey post hoc test.