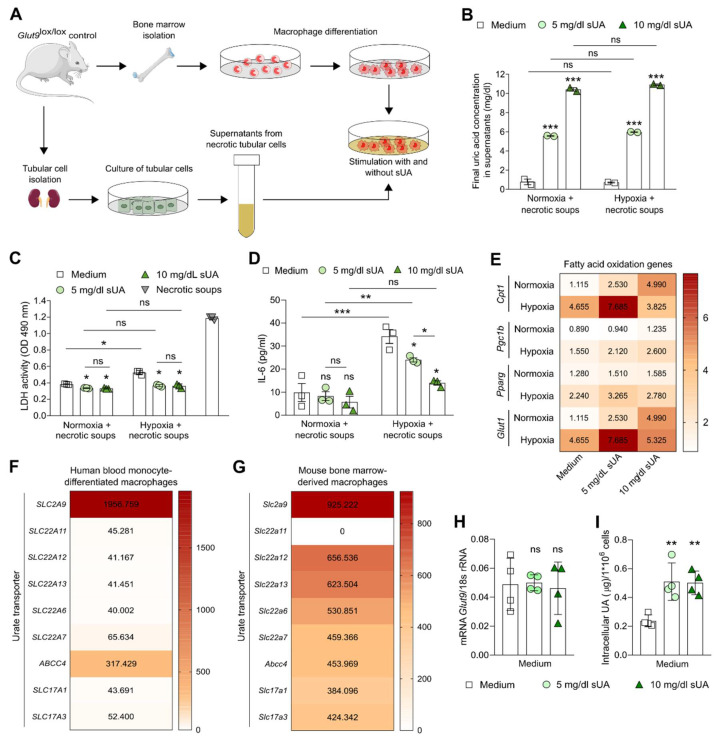
Figure 2.
Soluble uric acid inhibits the pro-inflammatory function but enhances the metabolic activity of macrophages under hypoxic and DAMP-related tubular injury condition. (A) Schematic of experimental setup. Bone marrow-derived macrophages from Glut9lox/lox mice were primed with LPS for 24 h prior to sUA stimulation (medium; 5 mg/dL sUA; 10 mg/dL sUA). To mimic ischemic/reperfusion injury in the kidney, cells were cultured under hypoxic (5% O2) and/or normoxic (21% O2) conditions with and without damage-associated molecular pattern (DAMP)-containing supernatants from necrotic tubular epithelial cells for 24 h. (B) Final UA concentrations after adding 5 and 10 mg/dL sUA into the macrophage culture were measured by colorimetric assay. (C) Cytotoxicity (LDH activity) of macrophages was assessed by LDH assay (absorbance, OD 490 nm) (n = 3). DAMP-containing supernatants from necrotic tubular epithelial cells were used as positive control (necrotic soups). (D) Concentrations of interleukin (IL)-6 in culture supernatants were determined by ELISA (n = 2–3). (E) mRNA expression levels of genes associated with fatty acid oxidation Cpt1, Pgc1b, Pparg, and Glut1 were determined by RT-qPCR (n = 4–5). Data are expressed as mean ± SEM. (F,G) Online available microarray data of urate transporters expressed by human blood monocyte-differentiated macrophages (F, n = 3) and mouse bone marrow-derived macrophages (G, n = 3) [38,39]. (H) mRNA expression of the UA transporter Slc2a9/Glut9 in mouse bone marrow-derived macrophages in the presence or absence of sUA determined by RT-PCR (n = 3). (I) Mouse bone marrow-derived macrophages with or without 5 or 10 mg/dL sUA were digested and the intracellular sUA concentration was measured (n = 4). * p < 0.05; ** p < 0.01; *** p < 0.001; ns, not significant; one- or two-way ANOVA with Tukey post hoc test.