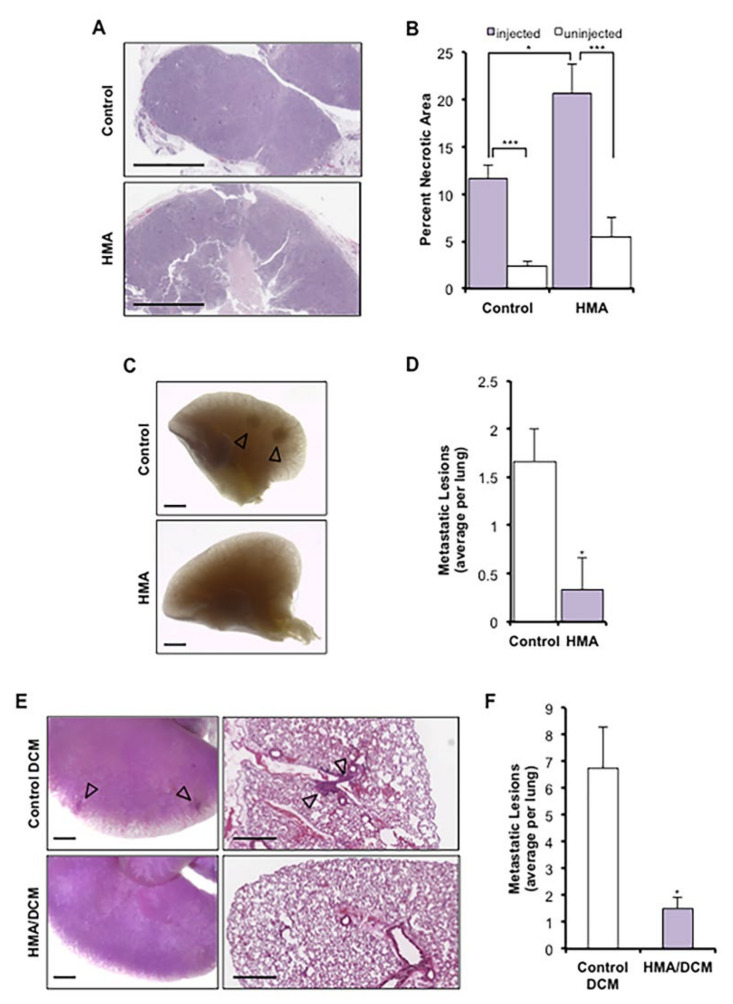
Figure 4.
HMA induces necrosis in vivo and suppresses metastasis to the lung. (A–D) Met-1 xenograft mammary tumors received daily intratumoral injection of HMA (0.78 μg/mm3 tumor volume) or vehicle for 7 days (n = 3 FVB/NJ mice per cohort). (A) Representative H&E-stained sections; scale bar = 1 mm. (B) Necrotic area is presented as a percentage of total tumor area (mean ± SEM) and compared to un-injected contralateral (internal animal control) tumors. Significance was determined by Student’s t-test. (C) Carmine alum-stained lung tissue was evaluated for the occurrence of gross lesions (arrows); scale bar = 1 mm. (D) The numbers of gross metastatic lesions per mouse (mean ± SEM) are depicted for each treatment cohort, and significance was determined by Student’s t-test. (E,F) NDL tumor cells were instilled into the lateral tail vein of FVB/NJ mice, and animals were treated intravenously twice weekly for four weeks with HMA (30 mg/kg)-loaded DCMs (HMA loading: 5 mg/mL) or empty DCM (n = 4 mice per cohort). (E) Metastatic colonization of the lungs was determined by carmine alum staining; scale bar = 1 mm. (F) Quantification of metastatic lung lesions per animal (mean ± SEM). Significance was determined by Wilcoxon rank-sum test with continuity correction. *, p < 0.05; ***, p < 0.001, for all panels.