Abstract
As populations age worldwide, the burden of valvular heart disease has grown exponentially, and so has the proportion of affected women. Although rheumatic valve disease is declining in high-income countries, degenerative age-related etiologies are rising. Calcific aortic stenosis and degenerative mitral regurgitation affect a significant proportion of elderly women, particularly those with comorbidities. Women with valvular heart disease have been underrepresented in many of the landmark studies which form the basis for guideline recommendations. As a consequence, surgical referrals in women have often been delayed, with worse post-operative outcomes compared to men. As described in this review, a more recent effort to include women in research studies and clinical trials has increased our knowledge about sex-based differences in epidemiology, pathophysiology, diagnostic criteria, treatment options, outcomes, and prognosis.
Keywords: Cardiovascular Disease, Valvular Heart Disease, Women, Sex, Gender
INTRODUCTION
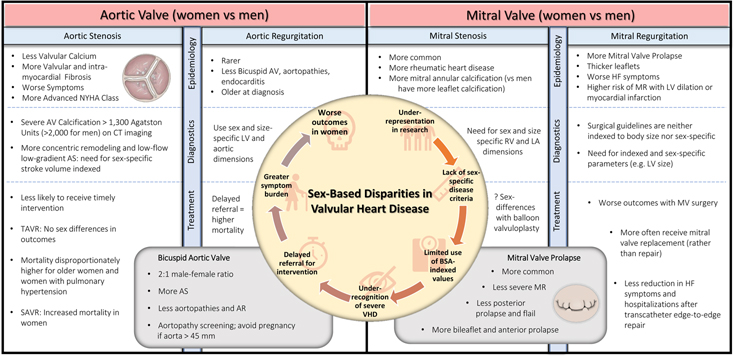
Valvular heart disease (VHD) increases with age, with the majority of VHD diagnosed in patients greater than 65 years old. In an echocardiographic study of 2,500 primary care patients over 65 years old in the United Kingdom, over half had some degree of VHD, while 6.4% had moderate to severe VHD.1 Men and women are equally likely to experience VHD, but sex-specific prevalence varies by type of valve lesion.1–3 In general, women more frequently suffer from mitral valve (MV) diseases such as mitral valve prolapse (MVP) or rheumatic MV disease, while men more often develop aortic valve (AV) diseases including aortic regurgitation or aortic stenosis (AS) associated with bicuspid AVs (Central Illustration, Figure 1 and Figures 2a–c). Men are also more likely than women to suffer from endocarditis of any valve.
Figure 1.
CENTRAL ILLUSTRATION: Sex Differences in Valvular Heart Disease
Figure 2.
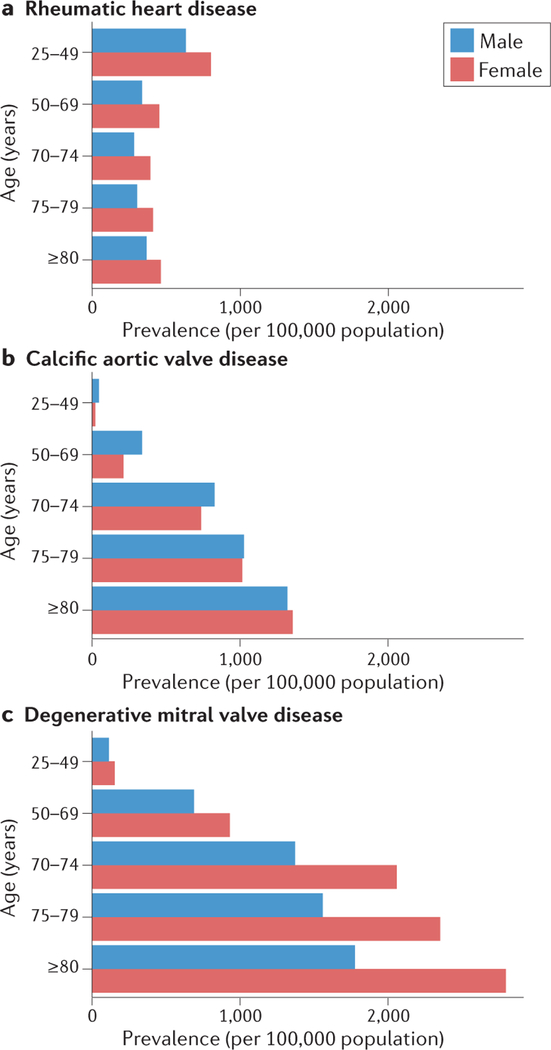
Age- and Sex-Specific Global Prevalence of Three Forms of Valvular Heart Disease2
The epidemiology of VHDs is shifting away from rheumatic- and congenital- etiologies and towards degenerative age-related etiologies.4 As the age at diagnosis continues to increase in patients with VHD, so does the proportion of women, making it increasingly important to understand how sex-based differences affect diagnosis, treatment, and prognosis in VHD. Women with VHD have been underrepresented in many of the landmark studies which form the basis for guideline recommendations. As a result, our understanding of the pathophysiology, clinical manifestations, diagnostic criteria, and management strategies are extrapolated from data on male patients. Important metrics for grading disease severity and gauging timing for surgery were developed in predominately male populations. Left ventricular (LV) dimensions in aortic and mitral regurgitation, poor adoption of parameters indexed to body size, and lack of sex-specific diagnostic criteria may all lead to inaccurate quantification of VHD severity for women who, among other differences, have smaller hearts than men.5 Sex-based disparities in the diagnosis of VHD may cause women to be more symptomatic and experience later referral for surgical or percutaneous interventions (Central Illustration, Figure 1).
Employing body-surface indexed measurements or sex-specific diagnostic criteria may decrease anatomical and biological sex-based disparities in VHD. However, psychological and societal factors continue to contribute to sex-based disparities in many aspects of cardiovascular care. Despite ongoing efforts to include more women in clinical trials,6 enrollment of women still lags behind disease prevalence for many conditions. In addition, women are less likely to receive prescriptions for certain evidence-based cardiovascular medications, and women have lower rates of referral for interventional procedures.7, 8 Patient-provider gender concordance has been shown to influence patient outcomes, yet women with VHD are also rarely cared for by female clinicians.9 Women comprise only about 15% of practicing cardiologists and 4% of interventional cardiologists.10, 11 Regardless of gender of the provider, physicians are known to underestimate female patients’ pain and attribute symptoms to mental rather than physical conditions. Throughout this review we outline how women with VHD often experience later referrals for valve replacement and worse outcomes. Gender disparities in VHD are almost certainly multifactorial with contributions from underappreciation of the physiologic differences in VHD between sexes, cultural and societal norms by gender, and unconscious bias from providers.
This review explores the current evidence on sex-based differences in VHD, including epidemiology, pathophysiology, diagnostic criteria, treatment options, outcomes, and prognosis.
AORTIC VALVE DISORDERS
Etiology and Prevalence
In developed countries, AV disease makes up the majority of VHD, with AS comprising 47% and aortic regurgitation comprising 18% of all VHD, respectively.3 Non-rheumatic, calcific AV disease affects over 12 million patients globally, and is the most prevalent VHD in high-income countries.12 Male sex has classically been considered a major risk factor for AS partly because of the higher prevalence of bicuspid AV in men. In a recent study of 25,556 newborns in Denmark screened with echocardiography, bicuspid AVs were identified in 0.77% of screened newborns with a male-female sex ratio of about 2 to 1.13 However, the majority of patients with AS in the United States now have calcific degeneration of tricuspid AVs. Although men are still at higher risk of developing AS overall than women, AS is increasingly common in elderly women and the majority of AS patients over 80 years old are women (Figure 2b).2, 3, 14–16 Similarly, the risk of moderate or greater aortic regurgitation also increases with age, reaching almost 2% in patients over 65 years old.1 Therefore, as with AS, the proportion of women with aortic regurgitation increases with age. Nonetheless, aortic regurgitation has more marked sex differences than AS and is more common among men across the age spectrum, possibly due to the male predilection for both bicuspid AV and endocarditis.3, 17
Pathophysiology
Male sex and sex aneuploidy (e.g. Turner syndrome) are well-established risk factors for congenital AV disease and aortopathies, leading to the hypothesis that X-linked genes are instrumental in the development of a normal aorta and AV.18 Familial clustering has been clearly demonstrated and hereditability has been estimated as high as 89%.16, 19 Genes associated with bicuspid AV include NOTCH1, GATA4, GATA6, SMAD4, SAMD6, and ROBO4.20–23 In addition, different variants may be associated with bicuspid AV according to sex: EGFR rs533525993 and TEX26 rs12857479 are linked to bicuspid AV in men and NOTCH1 rs61751489, TGFBR2 rs1155705, and NKX2–5 rs2277923 in women.24
The pathogenesis of acquired AS shares many similarities with vascular atherosclerosis, which also has a male predilection: endothelial damage results in lipid infiltration, inflammation, fibrosis, and calcification. In AS, leaflet calcification is considered the culprit lesion, leading to leaflet thickening and restriction.25 However, it is now well-established that women with severe AS have less AV calcium compared to men, even after indexing to body surface area or aortic annulus area.26–29 Sex-specific hormonal differences may explain why men develop more calcified AVs: apolipoprotein E-null mice treated with testosterone have greater calcific deposition in the aortic sinus.30 However, it remains unclear why women develop hemodynamically more severe AS at a lower degree of AV calcification. One potential explanation may be valvular fibrosis.31 In a study of 125 patients with explanted stenotic tricuspid AVs, women were found to have more histologically-quantified valvular fibrosis compared to men, despite having lower aortic calcification density. In a subgroup of 24 tightly-matched patients, valves explanted from women had more collagen fibers and a greater proportion of the valve occupied by dense connective tissue.31
Clinical Presentation
Women with severe AS more often have diabetes, hypertension, chronic lung disease, and atrial fibrillation, while men more often have coronary or peripheral arterial disease.32 Wild-type transthyretin cardiac amyloid has a high prevalence among patients with age-related degenerative AS—and affects about 15% of patients undergoing transcatheter aortic valve implantation (TAVI).33, 34 Wild type transthyretin amyloid cardiomyopathy disproportionately affects men, with a male proportion estimated up to 87%.35 At presentation, women with severe AS are often older and have greater symptom burden, including having more exertional dizziness and more advanced New York Heart Association (NYHA) class.36 Although less well-studied, a similar trend of greater symptomatology among women has also been described in aortic regurgitation.37 Among patients with bicuspid AVs, men are more likely to develop complications including aortic regurgitation, aortopathies, and endocarditis, while women more often develop AS.38
Optimal Imaging Modalities
Echocardiography is the primary modality for the assessment of AV morphology and function, as well as the effects of valvular pathology on cardiac chamber size and function. 39, 40 Transthoracic echocardiography (TTE) is the initial test of choice. However, transesophageal echocardiography (TEE) as well as computed tomography (CT) and cardiac magnetic resonance (CMR) imaging have become important adjuncts to the accurate diagnosis and management of AV disease. Reflecting basic science studies,30, 41 women with AS present with less AV calcification than men, as measured by echocardiography,42 histology27 and CT43 for the same hemodynamic severity of AS. Compared to men, women with AS had more pronounced fibrotic remodeling, irrespective of the valve morphology or age of the patient.44 Thus, thresholds of AV calcification used to identify severe AS are sex-specific, with 1,300 Agatston Units in women and 2,000 Agatston Units in men 28, and useful in differentiating significant disease in the setting of discordant echocardiographic grading (i.e. low flow, severe AS) (Table 1). Calcium burden predicts adverse outcomes42, 45 and thus may disproportionately affect men compared to women. However, two studies, COFRASA (Aortic Stenosis in Elderly: Determinant of Progression) and GENERAC (Genetic of Aortic Valve Stenosis-Clinical and Therapeutic Implications), used multi-slice CT to show the annualized relative progression rate was significantly higher in women (33 ± 32% vs. 19 ± 16%, p = 0.004). After adjusting for lower baseline CT calcium score, female sex was an independent predictor of AV calcium progression (p = 0.002).46
Table 1.
| Imaging parameters of severe valvular heart disease (ACC/AHA Guidelines) | Gender considerations in grading the severity of valvular heart disease | |
|---|---|---|
| Aortic Stenosis | • High-Gradient AS: Vmax ≥4 m/s, mean ΔP ≥40 mmHg, AVA ≤1.0 cm2 • Low-Flow Low-Gradient AS with Reduced LVEF: AVA ≤1.0 cm2, Vmax <4 m/s, mean ΔP <40 mmHg; Dobutamine stress echocardiography shows AVA <1.0 cm2 with Vmax ≥4 m/s at any flow rate • Paradoxical Low-Flow AS with Normal LVEF: AVA ≤1.0 cm2 with Vmax <4 m/s or mean ΔP <40 mm Hg AND stroke volume index <35 mL/m2 |
• Indexed AVA on 2D Echocardiography: ○  / / - Indexed AVA ≤ 0.6 cm2/m2 - Indexed AVA ≤ 0.6 cm2/m2• Gender-Specific Stroke Volume Index on 2D Echocardiography in Paradoxical Low-Flow Low-Gradient Aortic Stenosis: ○  - Stroke Volume Index ≤ 32 ml/m2 - Stroke Volume Index ≤ 32 ml/m2○ 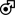 - Stroke Volume Index ≤ 40 ml/m2 - Stroke Volume Index ≤ 40 ml/m2• Quantification of AV Calcification on Cardiac CT: ○  - Severe Calcification > 1,300 Agatston Units - Severe Calcification > 1,300 Agatston Units○ 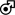 - Severe Calcification > 2,000 Agatston Units - Severe Calcification > 2,000 Agatston Units |
| Aortic Regurgitation | • Jet width ≥65% of LVOT • Vena contracta >0.6 cm • Holodiastolic flow reversal in the proximal abdominal aorta • Regurgitant volume ≥60 mL/beat • Regurgitant fraction ≥50% • EROA ≥0.3 cm2 • LV dilation |
• Gender-Specific and Indexed LV Dimensions on 2D Echocardiography: ○  - Normal: LV EDV index <61mL/m2, LVESV index < 35 mL/m2 - Normal: LV EDV index <61mL/m2, LVESV index < 35 mL/m2○  - Normal: LV EDV index <74mL/m2, LV ESV index < 24 mL/m2 - Normal: LV EDV index <74mL/m2, LV ESV index < 24 mL/m2○  / / - consider indexed LV ESD > 25 mm/m2 in addition to the non-indexed LV ESD >50 mm to define LV dilation - consider indexed LV ESD > 25 mm/m2 in addition to the non-indexed LV ESD >50 mm to define LV dilation• Gender-Specific and Indexed Aortic Dimensions on 2D Echocardiography or CMR: ○ See reference tables for Age and Gender normal ranges for aortic annulus, sinuses of Valsalva, sinotubular junction, and proximal ascending aorta sizes. • Gender-Specific and Indexed LV Chamber Size on CMR: ○  - Normal: LV EDV index < 96 mL/m2, LV ESV index < 34 mL/m2 - Normal: LV EDV index < 96 mL/m2, LV ESV index < 34 mL/m2○  - Normal: LV EDV index < 105 mL/m2, LV ESV index < 38 mL/m2 - Normal: LV EDV index < 105 mL/m2, LV ESV index < 38 mL/m2 |
| Mitral Stenosis | • Mitral valve area ≤1.5 cm2 • Diastolic pressure half-time ≥ 150 ms • Severe LA enlargement • Elevated pulmonary artery systolic pressure > 50 mmHg |
• Indexed LA Volumes on 2D Echocardiography: ○  / / - Normal: LA volume ≤ 34 mL/m2 - Normal: LA volume ≤ 34 mL/m2 |
| Mitral Regurgitation | • Central jet MR >40% LA or holosystolic eccentric jet MR • Vena contracta ≥0.7 cm • Regurgitant volume ≥60 mL • Regurgitant fraction ≥50% • EROA ≥0.40 cm2 • Moderate or severe LA enlargement • LV enlargement • Pulmonary hypertension |
• Gender-Specific and Indexed LV Dimensions on 2D Echocardiography or CMR: ○ See “Aortic Regurgitation” Section above • Indexed LA Volumes on 2D Echocardiography: ○ See “Mitral Stenosis” Section above |
| Tricuspid Regurgitation | • Central jet ≥50% RA • Vena contracta width ≥0.7 cm • EROA ≥0.40 cm2 • Regurgitant volume ≥45 mL • Dense continuous wave signal with triangular shape • Hepatic vein systolic flow reversal • Dilated RV and RA |
• Gender-Specific and Indexed RA Dimensions on 2D Echcardiography: ○  - normal RA volume 21 + 6 mL/m2 - normal RA volume 21 + 6 mL/m2○  - normal RA volume 25 + 7 mL/m2 - normal RA volume 25 + 7 mL/m2• Gender-Specific and Indexed RV Dimensions on 2D Echocardiography: ○  - Normal RV EDA index < 11.5 cm2/m2, RV ESV index < 6.4 cm2/m2, RV EDV index < 74 mL/m2, RV ESV index < 36 mL/m2 - Normal RV EDA index < 11.5 cm2/m2, RV ESV index < 6.4 cm2/m2, RV EDV index < 74 mL/m2, RV ESV index < 36 mL/m2○  - Normal RV EDA index < 12.6 cm2/m2, RV ESV index < 7.4 cm2/m2, RV EDV index < 87 mL/m2, RV ESV index < 44 mL/m2 - Normal RV EDA index < 12.6 cm2/m2, RV ESV index < 7.4 cm2/m2, RV EDV index < 87 mL/m2, RV ESV index < 44 mL/m2
• Gender-Specific RV Size and Function on 3D Echocardiography: ○ See reference tables for Age and Gender normal ranges for RV EDV, RV ESV, RV EF. Women have smaller volumes and slightly higher RV EF. • Gender-Specific and Indexed RV Chamber Size on CMR: ○  - Normal: RV EDV index < 112 mL/m2, RV ESV index < 52 mL/m2 - Normal: RV EDV index < 112 mL/m2, RV ESV index < 52 mL/m2○  - Normal: RV EDV index < 121 mL/m2, RV ESV index < 59 mL/m2 - Normal: RV EDV index < 121 mL/m2, RV ESV index < 59 mL/m2 |
| Pulmonic Stenosis | • Peak gradient 64 mmHg (peak velocity >4 m/s) • Mean gradient >35 mmHg • RV dilation and dysfunction |
• Gender-Specific RVOT Measurements on 2D Echocardiography: ○  - Normal RVOT EDA < 20 cm2 - Normal RVOT EDA < 20 cm2○  - Normal RVOT EDA < 24 cm2 - Normal RVOT EDA < 24 cm2
|
AS, aortic stenosis; AV, aortic valve; AVA, aortic valve area; ERO, effective regurgitant orifice; EDV, end-diastolic volume; EDA, end-diastolic area; ESD, end-systolic dimension; EF, ejection fraction; ESV, end-systolic volume; LA, left atrium; LV, left ventricular; ΔP, pressure gradient between the left ventricle and aorta; RA, right atrium; RV, right ventricular; RVOT, right ventricular outflow tract; Vmax, maximum velocity.
Sex differences in LV remodeling have also been described in patients with AS. Men more often have eccentric LV hypertrophy whereas women have concentric LV hypertrophy with greater diffuse intra-myocardial fibrosis detected by extracellular volume fraction on CMR (Central Illustration, Figure 1 and Table 1).47 Echocardiographic analysis showed that women were more likely to manifest heart failure with preserved ejection fraction with higher LV ejection fraction, higher LV mass index, and higher left atrial volume index.48 Importantly, concentric remodeling detected by echocardiography has been identified as a predictor of worse outcomes in women but not in men. 49 Concentric remodeling may result in low flow (≤35 ml/m2), low gradient AS; however, the applicability of this cutoff to both males and females may not be appropriate. Sex-specific thresholds of stroke volume index that are independently associated with increased mortality: for men the cutoff of 40 ml/m2 was associated with an adjusted hazard ratio (HR) of 1.54 (95% CI: 1.02 to 2.32, p = 0.042), and for women the cutoff of 32 ml/m2 was associated with an adjusted HR of 2.05 (95% CI: 1.21 to 3.47, p < 0.01) (Table 1).50
Percutaneous Treatment Options
TAVI has become the standard of care for patients with symptomatic severe AS at high and prohibitive risk for surgical intervention. In addition, current guidelines give TAVI a Class I indication for patients aged 65–80 years and characteristics suitable for transfemoral approach 40. Unlike coronary artery disease trials, women constitute almost half of the patients studied in trials. However, women demonstrate significant differences in baseline characteristics. They are older with fewer comorbidities, a finding consistent across randomized trials and registries alike 51, 52. Procedural outcomes of TAVI by gender show no differences in device success. Nonetheless, female gender is associated with an increased rate of major vascular complications and major bleeding in TAVI 51–53, albeit with a lower incidence of ≥moderate paravalvular regurgitation and multiple studies reporting better mid- 54 and long-term survival 55. Recent TAVI trials and registries 53, 56–58 show no apparent sex-specific differences in survival or stroke on multi-variable analysis, possibly reflecting the changing demographic of patients enrolled, use of newer-generation valves and delivery systems, and more accurate valve sizing techniques. Analysis of the Society of Thoracic Surgeons (STS) / American College of Cardiology registry found no sex-specific differences in post-TAVI health-related quality of life as measured by the Kansas City Cardiomyopathy Questionnaire.59
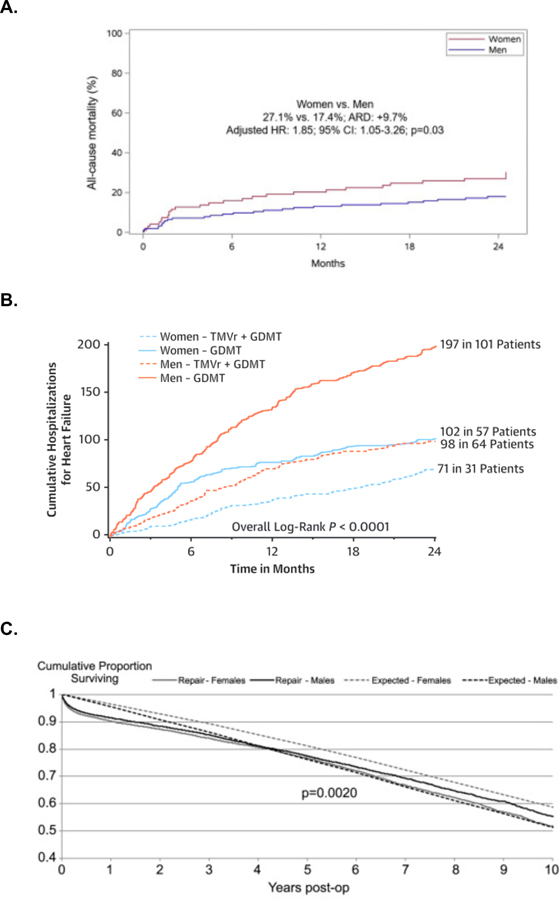
Nonetheless, mortality was found to be higher in older women compared to older men in the FRAILTY-AVR study. 48 Women in this study had higher levels of physical but not cognitive frailty at baseline, with a higher risk of 1-month mortality or major morbidity (the latter driven by vascular bleeding complications). Women undergoing TAVI in the FRAILTY-AVR cohort more frequently had physical frailty and more often required discharge to a rehabilitation facilities.48 Mortality at 12-months was greater in women with pulmonary artery systolic pressure >60 mmHg (Figure 3a). However, in the absence of pulmonary hypertension, there was no sex-specific differences in outcomes (Figure 3a). Similar findings were also seen in the PARTNER I study, in which pulmonary hypertension was associated with adverse outcomes in women but not men.60 Female sex is a well-established risk factor for pulmonary vascular disease, possibly due to multifactorial effects of estrogen on pulmonary vascular remodeling.61 Women with heart failure with preserved ejection fraction may also preferentially develop pulmonary hypertension, which is associated with increased symptoms and more right ventricular dilation.62 Similar pathophysiology may occur in valvular heart disease, in which women may be prone to more pulmonary vascular remodeling, more symptomatology, and worse outcomes. Although outcomes in TAVI are improving with time, gender disparities in TAVI persist.63
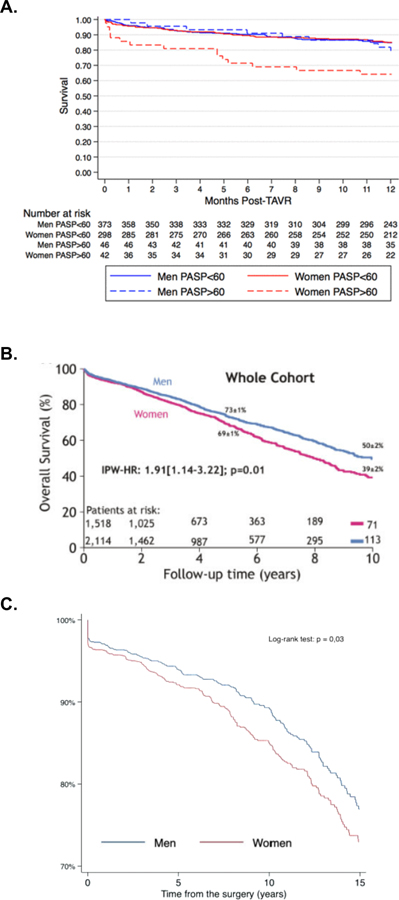
Figure 3:
Sex-Based Differences in Survival after Interventions for Aortic Stenosis
A) Survival after Transcatheter Aortic Valve Intervention
Survival following Transcatheter Aortic Valve Intervention among patients with and without pulmonary hypertension in FRAILTY-AVR48
B) Survival among patients with at least Mild-to-Moderate AS
Survival among patients with at least mild-moderate AS in single-center from Canada. IPW-HR, inverse-probability weighted hazard ratio65
C) Survival after Surgical Aortic Valve Replacement
Sex-Differences in Survival following Surgical Aortic Valve Replacement After Propensity Score Matching in the Multicenter Spanish Aortic Valve Registry68
Surgical Options
Recent analyses of clinical practice in Europe and the United States (US) show persistent undertreatment of AV disease compounded by sex disparities.64, 65 For example, in a US cohort of 43,000 patients diagnosed with severe AS between 2008 and 2016 from 2,000 hospitals and 7,000 outpatient clinics, only 28% of patients underwent surgical or transcatheter intervention within a year of diagnosis, and women were 20% less likely than men to undergo AV replacement, even after adjusting for clinical characteristics, socioeconomic status and access to healthcare.64 Women who did undergo intervention were more likely to be treated with TAVI compared to men.64 Similar findings were also shown in a retrospective analysis of 3,632 patients with at least mild-to-moderate AS from a single-center in Canada. Despite comparable hemodynamics to men and adjustment for covariates, women in this cohort experienced a higher risk of death over a mean of 4 years of follow up (Figure 3b). The worse survival among women was partially attributed to a lower rate of aortic valve replacement (surgical or transcatheter), especially among women with low-gradient aortic stenosis.65 In several analyses, women treated with surgical AV replacement (SAVR) were more likely to be older with more advanced disease at the time of surgery than men, and this is reflected in higher operative mortality after SAVR in women compared to men.64, 66, 67 Female sex per se’ has been also been reported to be an independent predictor of worse operative mortality and morbidity after SAVR (Figure 3c).64, 67, 68
Female sex is included as a risk factor in many surgical risk prediction tools such as the Society of Thoracic Surgeons (STS) and EuroSCORE calculators. These risk prediction scores are frequently used in clinical practice to select which patients are considered surgical candidates. These risk scores do have validity in women; for example, the EuroSCORE I was shown to be an independent predictor of 1-year death or stroke in 1,019 intermediate- to high-risk women with severe symptomatic AS in the WIN-TAVI registry, the first TAVI registry enrolling exclusively women.69 However, risk scores are often derived in populations of predominantly men and required continued validation in cohorts of women. Even the most updated version of the STS calculator was developed in a SAVR cohort comprised of only 40% women.70 Furthermore, while risk prediction calculators are important tools in the assessment of peri-operative risk, they are derived and validated from retrospective analyses of real-world populations and are therefore susceptible to referral bias. Women will always appear higher-risk surgical candidates relative to men on risk predictions tools, and it’s possible this may further promote disparities and further delay referral for consideration of surgical valve interventions.
Prognosis
The mortality of untreated severe AS at one year in an analysis of 43,000 US patients was similar for men and women (31.1% vs. 31.3%, adjusted HR 0.98, 95% confidence interval (CI) 0.94–1.03). 64 Intervention was associated with substantially improved prognosis, however one-year mortality after SAVR was significantly higher in women (9.0%) compared to men (7.6%) (HR 1.43, 95% CI 1.21–1.69). Unmeasured confounders such as increased frailty, more patient prosthesis mismatch with smaller aortic annular dimensions, higher prevalence of paradoxical low-flow aortic stenosis, and higher incidence of permanent pacemaker requirement in women may contribute to poorer short-term outcomes and worse long-term survival.65, 71, 72 These factors may have less impact on outcomes after TAVI, where there no significant difference in mortality exists between women and men at one year (HR 1.05, 95% CI 0.86–1.27).64 The ongoing RHEIA trial (NCT04160130), a randomized controlled trial of SAVR versus TAVI in women, will help further elucidate if outcomes for TAVI are superior to SAVR in women.
Implications for Pregnancy
Native Aortic Valve Disease
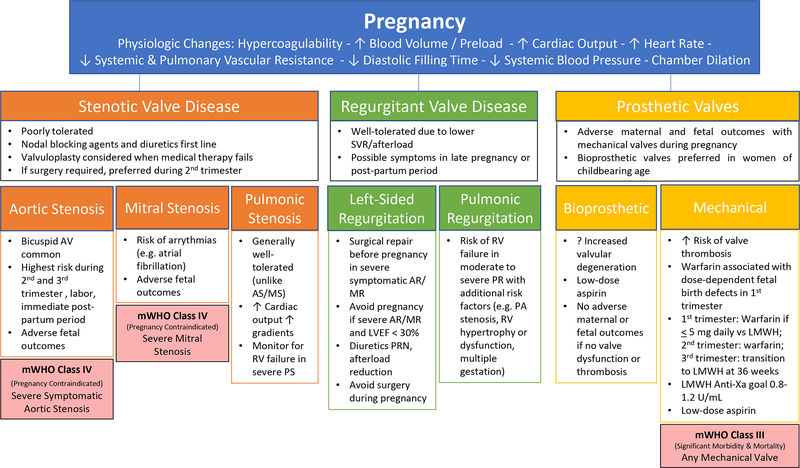
Pregnancy introduces physiological conditions that may impact AV disease. It is important to perform a comprehensive assessment of AV disease when considering pregnancy and implement a plan for cardiovascular monitoring throughout pregnancy, during delivery, and in the post-partum period. In general, AS is more problematic than aortic regurgitation during pregnancy. The additional pressure load is poorly tolerated, and there is the potential for significant morbidity for both the fetus and the mother. Patients with mild to moderate AS and aortic regurgitation are generally considered at moderate risk for morbidity and mortality according to the modified World Health Organization (WHO) classification and risk assessment.73 However, maternal risk can vary considerably within these categories, and algorithms exist for more individualized assessment during pregnancy, including the CARPREG II and ZAHARA models.74, 75
Patients with asymptomatic severe AS may be medically managed throughout pregnancy provided they do not meet Class of Recommendation I criteria for valve intervention and asymptomatic status has been confirmed with exercise testing. Women with symptomatic severe AS have a high risk of adverse maternal outcomes and are advised against pregnancy (Figure 4).73, 76 Patients with severe aortic regurgitation are at moderate risk but can generally be medically managed through pregnancy. Surgical intervention is typically necessary in patients who have NYHA class 4 symptoms and severe aortic regurgitation.40
Figure 4.
Special consideration should be given to patients with bicuspid AVs (regardless of presence of stenosis or regurgitation) and a dilated aorta. Patients with bicuspid AV and ascending aortic dimensions of <45 mm are considered to have moderate maternal risk of morbidity and mortality. Patients with bicuspid AV and aorta dimension of 45–50 mm are considered to have severely increased risk of morbidity and mortality. Women with aortic dimensions of >50 mm are advised against pregnancy.73
The complexity of these issues and the potential need for intervention highlights the importance of a multidisciplinary team and shared decision-making for patients with severe AV disease during pregnancy.
Prosthetic Valves in Pregnancy
Pregnancy is associated with a hypercoagulable state and pregnant patients are at increased risk for thrombosis, raising special issues for those with valve prostheses. Patients with bioprosthetic valves are at lower risk than those with mechanical prosthesis, and are generally managed with aspirin alone, starting in the second trimester.
Patients with mechanical valve prostheses are considered to be at significantly increased risk according to WHO Classifications (Figure 4).73, 77 Maternal complications include valve thrombosis, bleeding, thromboembolism and death. Risks of fetal complications are also significant. Studies have shown approximately 33% of women with mechanical heart valves have a serious complication (including fetal complications) during pregnancy. The main causes of morbidity are related to the need for anticoagulation during pregnancy. Warfarin is associated with dose-dependent fetal birth defects in the first trimester and may cause significant maternal bleeding in the peripartum. There is currently no optimal anticoagulation strategy for both mother and fetus. However, a widely used strategy for anticoagulation briefly outlined in Figure 4 attempts to take both fetal and maternal factors into account.76 Overall, close monitoring of the mother and fetus is necessary throughout pregnancy by a multidisciplinary team with both maternal-fetal and cardiology expertise.
MITRAL VALVE DISORDERS
Etiology and Prevalence
In developed countries, mitral valve (MV) diseases comprise about a quarter of VHD, with mitral regurgitation (MR) being much more common than mitral stenosis (MS).3 However, globally, MR is the most common VHD, affecting about 1–2% of the world’s population with a prevalence that increases to 7–9% among patients over 75 years of age.78 Rheumatic heart disease is largely responsible for the global burden of MV pathology, and is more common among women across all age groups (Figure 2a).2 Worldwide, nonrheumatic degenerative MV disease is also more common in women compared to men (265 versus 209 cases per 100,000), although men predominate among patients with nonrheumatic MV disease undergoing surgery.3, 79, 80 Other disorders of the MV, including MS and mitral valve prolapse (MVP) also have a female predilection (Figure 2c).3, 81 MVP affects 2–3% of the population and occurs more commonly in women.3, 81–83
Pathophysiology
Whereas FLNA has been identified as causing an X-linked form of MVP, mutations in DCHS1 and DZIP1 have been recently associated with the more common form of autosomal dominant MVP.84–86 Sex-related differences exist not only in the prevalence of MVP in the familial setting,83 but also in valvular morphologic features at the population level. In a study of over 8,000 patients with MVP, women were found to have thicker leaflets, less posterior prolapse, and less flail compared to men (Central Illustration, Figure 1).87 These findings may be attributable to sex-based differences in extracellular matrix remodeling which predispose women to more myxomatous valves with bileaflet MVP, and men to posterior MVP with flail. Differences in valve morphology likely explain why women with MVP also less frequently have severe MR (10% vs 23%, p<0.001).87 In secondary MR, women seem at disproportionately elevated risk of developing MR as a consequence of myocardial infarction or coronary artery disease.16 Gender differences also exist in the pathophysiology of mitral apparatus calcification. Men may have more posterior leaflet calcification while female gender has been associated with increased risk of mitral annular calcification.88, 89
Clinical Presentation
Women with LV dilation or myocardial infarction may be at higher risk of developing MR compared to men.16 Compared to men, women referred for MV surgery for secondary MR more often have co-morbid hypertension and less often have ventricular arrythmias or a history of smoking.90 At time of referral, women have less dilated ventricles and less severe MR but may have worse heart failure symptoms and worse mental health scores.90 As seen in AV disease, pulmonary hypertension relating to MS seems to disproportionately affect women compared to men. Compared to men, women with mitral stenosis have more pre-capillary pulmonary hypertension which may be a function of adverse pulmonary vascular remodeling.91
Optimal Imaging Modalities
Echocardiography remains the primary imaging modality for assessment of the MV, providing assessment of the valve anatomy and function as well as the mechanism of abnormalities. Echocardiography has superior temporal resolution compared to CMR and CT, and is often more effective at assessing leaflet function and mobile masses. Doppler echocardiography provides valuable measures of valve hemodynamics such as stenosis and regurgitation. Echocardiography is generally easily accessible and widely available. It is important to consider sex- and size-specific parameters for LV size when evaluating for LV dilation in the setting of MR (Table 1).
TEE with 3D assessment is often necessary for more precise delineation of mitral valve pathologic anatomy, such as the mechanism of flail leaflet for decision-making regarding intervention. TEE can also be very helpful if the TTE image quality is poor, or if better spatial resolution is necessary to assess for endocarditis. CMR may also be helpful in these settings, particularly for quantification of MR. TEE is the primary imaging modality for both pre-procedure assessment and guidance of percutaneous MV procedures including repair and replacement. Real-time imaging for transseptal puncture, guidance of device, and post-deployment assessment are also critical roles of TEE.
CT has a more limited role in assessment of MV disease. There has been increased use of multidetector CT to assess MV annulus anatomy and neo-LV outflow tract anatomy in planning transcatheter MV replacement.92
Sex Differences in Procedural Options for Mitral Valve Disease
Although the prevalence of MVP is greater in women than men, men are referred for surgery or intervention at a higher rate.82, 93 It is therefore important to understand that current guidelines for intervention for primary MR are based primarily on a predominantly male population. Cut-off values are not consistently derived from indexed values nor on sex-based differences, which may also explain why women are under referred. In part due to delayed referral, female gender has been associated with worse outcomes after MV surgery (Central Illustration, Figure 1 and Figure 5a).94 In a post-hoc analysis of Cardiothoracic Surgical Network Clinical Trials on Ischemic Mitral Regurgitation randomized clinical trials, women had worse outcomes following surgery for ischemic MR (Figure 5b). In this study, women also had significantly worse functional status and quality of life at 2 years, as measured by multiple metrics 90 The reasons for this are unclear, although it has been hypothesized that there is a mismatch between degree of MR and ventricular size.90 Female gender is a strong predictor of reverse LV remodeling following transcatheter edge-to-edge repair (TEER).95 Nonetheless, women treated with TEER may experience a less marked reduction in heart failure hospitalizations compared to men (Figure 5c).96 As with SAVR, female sex is considered a risk factor in risk prediction tools such as the STS for surgical mitral valve replacement.70
Figure 5.
Sex-Based Differences in Outcomes after Interventions for Mitral Regurgitation
A) Mitral Valve Surgery for Ischemic Mitral Regurgitation
Sex-Differences in All-Cause Mortality following Mitral Valve Surgery for Severe Ischemic Mitral Regurgitation in the Cardiothoracic Surgical Trials Network90
B) Transcatheter Edge-to-Edge Repair
Sex-Differences in Heart Failure Hospitalizations following Transcatheter Edge-to-Edge Repair in the COAPT trial96
C) Surgical Mitral Valve Repair
Sex-Differences in Survival Following Surgical Mitral Valve Repair in Medicare Beneficiaries112
Although women make up the majority of patients with rheumatic MS, reports on outcomes after percutaneous balloon mitral valvuloplasty are mixed. Some studies report more favorable outcomes in men while other report more favorable outcomes in women, and it remains unclear if there are sex-based differences in outcomes after balloon valvuloplasty.97 For calcific MS, women are again more commonly affected (Figure 2c).88 Treatment options remain poor, although transcatheter therapy is being studied actively. It is likely that TEE and CT will be important in the assessment of suitability and guidance for calcific MS interventions as these therapies are developed.
Percutaneous Treatment Options
Current ACC/AHA guidelines give a IIA recommendation for TEER for MR to treat either severely symptomatic primary MR patients at high or prohibitive surgical risk, or secondary MR patients who are severely symptomatic despite guideline-directed medical therapy, in anatomically-appropriate MV anatomies. The ideal anatomy for TEER 98 includes: non-commissural pathology, no or minimal calcium in the grasping zone, baseline MV area ≥4 cm2, baseline transmitral gradient <4 mmHg, flail width <15 mm and flail gap <10 mm, coaptation depth <11 mm and coaptation length ≥2 mm.99 Patients with non-ideal anatomy may still benefit from TEER. 100–103 Sex differences in MV morphology have been described; women more often have anterior or bileaflet prolapse and men present more often with posterior leaflet prolapse. 82 Arrhythmogenic MVP is more common in young adult women, with distinctive mitral annular disjunction and systolic curling, associated with fibrosis of the papillary muscles and inferobasal LV. 104 Finally, female sex is a predictor of incident mitral annulus calcification 88 and 68% of patients in the transcatheter mitral valve replacement in Mitral Annulus Calcification Global Registry were women 105.
For secondary MR, additional criteria should be met prior to consideration of TEER therapy; the LV ejection fraction between 20–50%, LV end-systolic dimension ≤70 mm and pulmonary artery systolic pressure ≤70 mmHg. 40 These recommendations are based on the entry criteria for the COAPT trial. 106 However women represented only 36% of patients in COAPT and only 25% of patients in the Percutaneous Repair with the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation (MITRA-FR) trial 107, raising questions about the applicability of results to women. In a sub-analysis of the COAPT trial specifically looking at sex-specific outcomes, significant differences in baseline characteristics were seen; women were younger than men and had fewer comorbidities, but reduced quality of life and functional capacity at baseline.96 In a joint frailty model accounting for the competing risk of death, the 2-year cumulative incidence of the primary endpoint of all heart failure hospitalizations was higher in men compared with women treated with guideline directed medical therapy alone. The relative reduction in heart failure hospitalizations with TEER on the other hand, was greater in men (HR 0.43; 95%CI 0.34–0.54) than women (HR 0.78; 95%CI 0.57–1.05) (Pinteraction = 0.002). A significant interaction was present between time and treatment with TEER versus medical therapy alone for all heart failure hospitalizations in women (HR 0.57; 95%CI 0.39–0.84, and HR 1.39; 95%CI 0.83–2.33 between 0–1 year and 1–2 years after randomization, respectively, Pinteraction = 0.007). This interaction was not demonstrated in men (Pinteraction = 0.16). Female sex was independently associated with a lower adjusted risk of death at 2 years (HR 0.64; 95%CI 0.46–0.90; P = 0.011). Thus, although TEER resulted in improved clinical outcomes compared with guideline-directed medical therapy alone, irrespective of sex, the impact of TEER in reducing heart failure hospitalizations was less pronounced in women compared with men beyond the first year after treatment (Figure 5c).
Studies disagree about sex-differences in clinical improvement with the TRAMI registry 108 and the GRASP registry 109 demonstrating less improvement in NYHA Class, but the European Registry of Transcatheter Repair for Secondary Mitral Regurgitation (EuroSMR) study showing equivalent quality of life and symptomatic improvements in both women and men 110.
Surgical Options
Major sex disparities have been observed in the treatment of MV disease. In a national analysis of over 100,000 patients admitted to hospital with MR in France between 2014 and 2015, only 8% of patients underwent MV surgery within one year, and women were significantly less likely than men to undergo MV intervention.111 Women undergoing MV intervention do so later in the disease course: in a US cohort of 47,602 patients aged 65 years and older undergoing MV surgery between 2000 and 2009, 58% of women compared with 47% of men had preoperative heart failure in the year before surgical MV repair, and women were also more likely to have pre-operative atrial fibrillation, respiratory failure or need urgent surgery than men.112 For primary or degenerative MR, MV repair is preferred to replacement for early and late survival benefit.113 However, women undergoing mitral surgery were less likely than men to receive mitral repair rather than replacement (31.9% vs. 44%, p=0.001).112 These differences were reflected in higher mortality after MV surgery in women (7.7%) compared to men (6.1%) (odds ratio 1.24, 95%CI 1.14–1.36, P=0.0001).112, 114 Unmeasured confounders such as frailty, and differences in the prevalence and severity of secondary MR, rheumatic valve disease and mitral annular calcification may contribute to these differences.
Prognosis
In patients hospitalized with MR who did not undergo intervention, one-year mortality was 14.3%.111 Compared with a US population matched for age and sex, surgical mitral repair restored normal life expectancy for men, but not women.112 Long-term survival after MV repair was slightly worse for women (Figure 5a), although survival after MV replacement was similar between women and men at ten years (HR 0.99; 95%CI 0.96–1.02).112 There is no long-term population-level survival data yet available for percutaneous mitral repair or replacement.
Implications for pregnancy
Native Mitral Valve Disease in Pregnancy
Pregnancy results in increased circulating blood volume, increased cardiac output, and increased heart rate, all of which increase transvalvular gradients and arrythmia risk in MS.115 Severe MS is poorly tolerated in pregnancy, and is considered a contraindication to pregnancy by the modified WHO classification system (Figure 4).73 Pre-pregnancy intervention for severe MS is preferred whenever possible, and exercise stress testing is often useful in cases where MS severity or symptomatology is unclear. Activity restriction, beta-blockers (e.g. metoprolol, bisoprolol), and diuretics may be useful in symptomatic pregnancy women with MS, and anticoagulation may be required for women with co-occurring diagnosis of atrial fibrillation.73 Pregnant women with severe MS with favorable anatomy should be considered for percutaneous mitral balloon valvulotomy after 20 weeks gestation, which is associated with a lower rate of fetal complications relative to open mitral valve commissurotomy.116
Conversely, mitral regurgitation is generally well-tolerated during pregnancy. Physiologic chamber dilation during pregnancy may lead to annular dilation; however, reductions in systemic vascular resistance and afterload can often result in a net reduction of MR severity during pregnancy (Figure 4). Nonetheless, severe MR can be associated with poor outcomes in pregnancy when it is accompanied by significant reductions in LV function.73, 115
Prosthetic Valves in Pregnancy
Patients with bioprosthetic valves in the mitral position are at lower risk than those with mechanical prostheses, and are generally managed with aspirin alone, starting in the second trimester. Those with mechanical prostheses requiring anticoagulation are managed with a protocol similar to the one described for aortic mechanical prostheses (Figure 4).
TRICUSPID VALVE DISORDERS
Etiology and Prevalence
Tricuspid regurgitation (TR) may be due to primary or secondary etiologies. Primary TR includes a variety of congenital and genetic anomalies (e.g. Ebstein’s anomaly, tricuspid dysplasia, and myxomatous degeneration leading to tricuspid valve prolapse) or acquired valve disease (e.g., endocarditis, carcinoid, rheumatic involvement, or device-related). Secondary TR occurs when valvular anatomy is normal but there is incomplete leaflet coaptation due to tethering or tenting of the leaflets or annular dilation often from right ventricular dilation or dysfunction.117 In the Framingham Heart Study, mild or greater TR was identified in 18% of women, with a male-to-female ratio of 1 to 1.6,17 and a female preponderance for TR has been replicated in other community-based cross sectional studies.118 Nonetheless, specific etiologies including congenital TR, right-sided endocarditis, carcinoid heart disease, and device-related TR may be more common in men.18, 119, 120
Pathophysiology
Recently, there has been growing recognition that the tricuspid valve exhibits a great deal of normal anatomic variability, with only about half of patients having three well-defined leaflets and 39% having four functional leaflets.121 It remains unknown whether there are sex-related differences in tricuspid valve (TV) anatomy. However, women are significantly more likely than men to have TR, and once they develop mild regurgitation they progress more rapidly to moderate to severe disease (Table 2).122 One potential explanation is sex-related differences in annular anatomy. In a postmortem study of patients with secondary TR, myocardium was found in all male atrioventricular annuli, but consistently absent from female annuli. Consequently, the atrioventricular annuli of men were more elastic, more cellular, and smaller when correcting for heart weight.123 Therefore, it has been proposed that triggers such as atrial fibrillation have a more pronounced effect in driving annular dilation and secondary TR in women.
Table 2.
Sex Differences in Tricuspid Regurgitation
Women 
|
Men 
|
|---|---|
| • Generally more common (male-female ratio 1 to 1.6) • Progresses more rapidly • Atrial fibrillation confers greater risk • More symptomatic • Older at diagnosis |
• More often due to congenital lesions, endocarditis, device-related, due to LV dysfunction • Annuli more often contain myocardium (possibly more elastic, cellular, and smaller when correcting for heart weight) |
Clinical Presentation
Women with secondary TR present older and more symptomatic than men.124, 125 Although increasing pulmonary hypertension severity confers equal risk of TR to both sexes, atrial fibrillation seems to confer a greater risk of secondary TR in women compared to men (Table 2).125
Optimal imaging modalities for diagnosis
Echocardiography remains the first diagnostic test for the assessment of the TV. Visualizing of the TV should be performed from multiple TTE and TEE windows. The newest American Society of Echocardiography (ASE) guidelines outline the recommended TTE views for performing a comprehensive evaluation of the valve 126, assessment of function 127, and measurement of the right heart chamber.128 TEE is ideal for imaging the high variability of leaflet number and subvalvular complexity. A recent TEE study of 579 patients confirmed these finding with 3 leaflets seen in just over 50% of patients and 4 leaflets seen in 39% of patients 121. A majority of quadricuspid valves (32% of the entire cohort) had 2 posterior leaflets.121 The approach to 3D imaging of the TV has been addressed in both guidelines 129 and reviews.130
Grading the severity of TR has been well-described by the ASE guidelines 131 as well as the European Association of Echocardiography guidelines, and focuses on assessment by TTE 132. Multiple investigators are attempting to refine133–135 and validate136–139 newer methods and criteria for quantitation of TR. In addition, an extended grading scheme identifying 2 grades above severe,135 has recently been supported in the new ESC/EACTS Valvular Heart Disease guidelines.140
Multi-modality imaging of the tricuspid valve includes the use of CT as well as CMR to assess the TV apparatus (valvular annular, and subvalvular), right heart size and function, adjacent anatomic structures such as the right coronary artery and vena cava.141, 142 As with MR, evaluation TR severity often relies on quantification of chamber sizes. Just as LV dimensions are influenced by body size and sex, the same is true for right-sided chambers. Sex-specific RV dimensions for 2D echocardiography, 3D echocardiography, and CMR are available and may be references to allow for accurate quantification of chamber size and thus TR severity in women (Table 1).128, 143
Percutaneous Treatment Options
There are currently no transcatheter devices approved for use in the US, however 3 devices are currently in pivotal trials. Among the compassionate use or early feasibility trials, 144–146 women make up 53–66% of enrolled patients highlighting the sex-differences in prevalence of the disease in this elderly population.17 Women are diagnosed with significant TR at an older age compared with men (72 [62 to 79] years vs. 70 [61 to 77] years; p = 0.003) and the TR etiology in women was more often isolated or related to left-sided VHD, whereas men more often had LV dysfunction related TR.124 After propensity score matching, there was no sex difference in outcomes, however TR etiologies remained significantly associated with all-cause mortality with left valvular disease or LV dysfunction related TR had lower survival compared with patients with primary TR (p = 0.004 and p = 0.019, respectively).
Surgical Options
Surgery for isolated TV disease is rarely performed: in a state-wide analysis of 289 patients diagnosed with moderate to severe or severe TR, only 3.8% of the study population underwent tricuspid operation during the 10-year study period.147 An analysis of the National Inpatient Sample, which represents approximately 20% of all US hospital admissions, identified between 290 and 780 operations per year in the United States between 2004 and 2013.148 In this analysis in-hospital mortality after tricuspid surgery was 8.8%, repair was associated with better survival and there was no significant difference in outcomes between men and women, who represented the majority of patients undergoing tricuspid surgery (58%) (Table 2).
Prognosis
The prognosis of TV disease is predominantly determined by the severity of left-sided heart disease. In a state-based analysis of 289 patients diagnosed with moderate to severe or severe TR, five-year mortality was significantly higher (47.8%) compared to an age and sex-matched population (36.3%, P=0.005).147 Women with TR have more rapid progression from milder disease to moderate and severe disease (Table 2).122 Sex-specific data on procedural outcomes in TR are limited, and sex-based differences have not been well-documented. Several risk prediction calculators have been proposed to help clarify the risk profile of patients being considered for tricuspid valve surgery. These risk prediction models, such as the TRI-SCORE, rely heavily on severity of heart failure severity (e.g. NYHA functional class, diuretic dose, LV and/or RV dysfunction, etc.) and co-morbidities (age, renal function, liver function).149, 150 The TRI-SCORE was developed from a cohort comprising of about 50% women; however, given women present with TR older and more symptomatic than men, further investigation is warranted on how risk prediction scores might affect surgical referrals by sex.
Implications for Pregnancy
The most common etiologies for TR in pregnancy are congenital abnormalities of the TV such as Ebstein’s Anomaly or atrioventricular canal defects, endocarditis, or rarely rheumatic valve disease. TR, even severe, in pregnancy is generally well tolerated.73
Pulmonic Valve Disorders
Over 95% of cases of pulmonic stenosis (PS) occur due to congenital defects. The prevalence of pulmonary valve disorders in adult patients are increasing due to an aging population as well as advances in the treatment of children with congenital heart defects. The best studied genetic contribution to PS is Noonan syndrome, and neither Noonan syndrome nor PS are thought to have a sex predilection.151, 152 Intervention with balloon valvuloplasty is typically required for PS in patients with gradients above 50 mmHg and current guidelines define severe PS as having peak gradient over 64 mmHg or mean gradient >35 mmHg on TTE (Table 1).113, 153 Early studies on balloon valvuloplasty did not investigate differences in outcomes by sex.154 More recent studies have not found differences in procedural outcomes by sex, although larger studies would be needed to confirm these findings.155 Pulmonic regurgitation (PR) most often results as a consequence of prior interventions on the pulmonic valve, and is generally well-tolerated. Nonetheless, of particular importance to women is the management of pulmonic valvular disease in pregnancy, given both PS and PR occur in women of childbearing age due to congenital abnormalities. Mild and moderate PS are well tolerated in pregnancy with little or no maternal risk (Figure 4).156 Severe PS is associated with increased maternal risk including preeclampsia, early delivery, thromboembolism, right ventricular failure and arrythmias.157 Management of pregnancy in patients with severe PS should be performed with a multidisciplinary heart valve team with consideration of pre-pregnancy intervention on the pulmonary valve. Pulmonary regurgitation, even severe, is well-tolerated in pregnancy. Intervention on the pulmonary valve may be needed in rare cases of severe symptomatic PR with RV failure.
GAPS IN KNOWLEDGE
Major gaps in knowledge still exist regarding sex-differences in VHD. Although the availability of advanced imaging techniques has grown exponentially, and “heart team” evaluations have become the standard of care in most medical centers, access to care may still be limited for women due to socioeconomic factors, racial disparities, or family obligations. As the primary caregivers for children and/or parents, women may defer clinical follow-ups or surgical interventions. The impact of social and psychological factors on long-term outcomes in women with VHD is unclear.
As highlighted by the most recent update of the ACC/AHA guidelines for the management of VHD,113 basic science studies on the genetic and pathobiological causes of valvulopathies are needed to better understand mechanisms of disease, and develop medical therapies to halt progression. Such studies should be enriched by female animal models to highlight sex-differences in biological pathways and response to therapy.
CONCLUSIONS
The burden of VHD has increased significantly among aging populations, and so has the proportion of women affected by VHD. Although men are still at higher risk of developing calcific AS than women, the majority of AS patients over 80 years old are women. Women with AS typically have less calcification and more fibrotic remodeling of the aortic valve, highlighting the importance of sex-specific thresholds of AV calcification by cardiac imaging. MV disease in women is more commonly related to MVP or rheumatic involvement. Left ventricular dimension values guiding surgical timing are not consistently based on indexed values nor on sex-based differences, which may explain why females are under-referred or experience delayed referrals and worse post-operative outcomes compared to men (Central Illustration, Figure 1). Overall, transcatheter therapies are becoming more available and appear to decrease sex differences in outcomes.
SOURCES OF FUNDING
This work was supported by the UCSF Cardiology Innovation Award and by the National Institutes of Health NHLBI R01HL153447 (Dr Delling).
ABBREVIATIONS
- ACC/AHA
American College of Cardiology / American Heart Association
- AS
Aortic Stenosis
- AV
Aortic Valve
- CMR
Cardiac Magnetic Resonance Imaging
- CT
Computed Tomography
- HR
Hazard Ratio
- LV
Left Ventricular
- MR
Mitral Regurgitation
- MS
Mitral Stenosis
- MV
Mitral Valve
- MVP
Mitral Valve Prolapse
- NYHA
New York Heart Association
- PS
Pulmonic Stenosis
- SAVR
Surgical Aortic Valve Replacement
- TAVI
Transcatheter Aortic Valve Intervention
- TEE
Transesophageal Echocardiogram
- TEER
Transcatheter edge-to-edge repair
- TR
Tricuspid Regurgitation
- TTE
Transthoracic Echocardiogram
- US
United States
- VHD
Valvular Heart Disease
- WHO
World Health Organization
Footnotes
DISCLOSURES
Dr. Hahn reports speaker fees from Abbott Structural, Baylis Medical, and Edwards Lifesciences; institutional educational and consulting contracts for which she receives no direct compensation with Abbott Structural, Boston Scientific, Edwards Lifesciences, Medtronic; equity with Navigate; and is Chief Scientific Officer for the Echocardiography Core Laboratory at the Cardiovascular Research Foundation for multiple industry-sponsored trials, for which she receives no direct industry compensation.
REFERENCES
- 1.d’Arcy JL, Coffey S, Loudon MA, Kennedy A, Pearson-Stuttard J, Birks J, Frangou E, Farmer AJ, Mant D, Wilson J, et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J. 2016;37:3515–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coffey S, Roberts-Thomson R, Brown A, Carapetis J, Chen M, Enriquez-Sarano M, Zühlke L and Prendergast BD. Global epidemiology of valvular heart disease. Nat Rev Cardiol. 2021;18:853–864. [DOI] [PubMed] [Google Scholar]
- 3.Andell P, Li X, Martinsson A, Andersson C, Stagmo M, Zöller B, Sundquist K and Smith JG. Epidemiology of valvular heart disease in a Swedish nationwide hospital-based register study. Heart. 2017;103:1696–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 5.Nitsche C, Koschutnik M, Kammerlander A, Hengstenberg C and Mascherbauer J. Gender-specific differences in valvular heart disease. Wien Klin Wochenschr. 2020;132:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin X, Chandramouli C, Allocco B, Gong E, Lam CSP and Yan LL. Women’s Participation in Cardiovascular Clinical Trials From 2010 to 2017. Circulation. 2020;141:540–548. [DOI] [PubMed] [Google Scholar]
- 7.Scott PE, Unger EF, Jenkins MR, Southworth MR, McDowell TY, Geller RJ, Elahi M, Temple RJ and Woodcock J. Participation of Women in Clinical Trials Supporting FDA Approval of Cardiovascular Drugs. J Am Coll Cardiol. 2018;71:1960–1969. [DOI] [PubMed] [Google Scholar]
- 8.Zhao M, Woodward M, Vaartjes I, Millett ERC, Klipstein-Grobusch K, Hyun K, Carcel C and Peters SAE. Sex Differences in Cardiovascular Medication Prescription in Primary Care: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2020;9:e014742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau ES, Hayes SN, Volgman AS, Lindley K, Pepine CJ and Wood MJ. Does Patient-Physician Gender Concordance Influence Patient Perceptions or Outcomes? J Am Coll Cardiol. 2021;77:1135–1138. [DOI] [PubMed] [Google Scholar]
- 10.Burgess S, Shaw E, Ellenberger K, Thomas L, Grines C and Zaman S. Women in Medicine: Addressing the Gender Gap in Interventional Cardiology. J Am Coll Cardiol. 2018;72:2663–2667. [DOI] [PubMed] [Google Scholar]
- 11.Wang TY, Grines C, Ortega R, Dai D, Jacobs AK, Skelding KA, Mauri L and Mehran R. Women in interventional cardiology: Update in percutaneous coronary intervention practice patterns and outcomes of female operators from the National Cardiovascular Data Registry®. Catheter Cardiovasc Interv. 2016;87:663–8. [DOI] [PubMed] [Google Scholar]
- 12.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sillesen AS, Vøgg O, Pihl C, Raja AA, Sundberg K, Vedel C, Zingenberg H, Jørgensen FS, Vejlstrup N, Iversen K, et al. Prevalence of Bicuspid Aortic Valve and Associated Aortopathy in Newborns in Copenhagen, Denmark. JAMA. 2021;325:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eveborn GW, Schirmer H, Heggelund G, Lunde P and Rasmussen K. The evolving epidemiology of valvular aortic stenosis. the Tromsø study. Heart. 2013;99:396–400. [DOI] [PubMed] [Google Scholar]
- 15.Toyofuku M, Taniguchi T, Morimoto T, Yamaji K, Furukawa Y, Takahashi K, Tamura T, Shiomi H, Ando K, Kanamori N, et al. Sex Differences in Severe Aortic Stenosis - Clinical Presentation and Mortality. Circ J. 2017;81:1213–1221. [DOI] [PubMed] [Google Scholar]
- 16.Fleury MA and Clavel MA. Sex and Race Differences in the Pathophysiology, Diagnosis, Treatment, and Outcomes of Valvular Heart Diseases. Can J Cardiol. 2021;37:980–991. [DOI] [PubMed] [Google Scholar]
- 17.Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL, Lehman B and Benjamin EJ. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol. 1999;83:897–902. [DOI] [PubMed] [Google Scholar]
- 18.Warnes CA. Sex differences in congenital heart disease: should a woman be more like a man? Circulation. 2008;118:3–5. [DOI] [PubMed] [Google Scholar]
- 19.Cripe L, Andelfinger G, Martin LJ, Shooner K and Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol. 2004;44:138–43. [DOI] [PubMed] [Google Scholar]
- 20.Bravo-Jaimes K and Prakash SK. Genetics in bicuspid aortic valve disease: Where are we? Prog Cardiovasc Dis. 2020;63:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison OJ, Visan AC, Moorjani N, Modi A, Salhiyyah K, Torrens C, Ohri S and Cagampang FR. Defective NOTCH signaling drives increased vascular smooth muscle cell apoptosis and contractile differentiation in bicuspid aortic valve aortopathy: A review of the evidence and future directions. Trends Cardiovasc Med. 2019;29:61–68. [DOI] [PubMed] [Google Scholar]
- 22.Lee A, Wei S and Schwertani A. A Notch more: Molecular players in bicuspid aortic valve disease. J Mol Cell Cardiol. 2019;134:62–68. [DOI] [PubMed] [Google Scholar]
- 23.Gould RA, Aziz H, Woods CE, Seman-Senderos MA, Sparks E, Preuss C, Wünnemann F, Bedja D, Moats CR, McClymont SA, et al. ROBO4 variants predispose individuals to bicuspid aortic valve and thoracic aortic aneurysm. Nat Genet. 2019;51:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dargis N, Lamontagne M, Gaudreault N, Sbarra L, Henry C, Pibarot P, Mathieu P and Bossé Y. Identification of Gender-Specific Genetic Variants in Patients With Bicuspid Aortic Valve. Am J Cardiol. 2016;117:420–6. [DOI] [PubMed] [Google Scholar]
- 25.Zheng KH, Tzolos E and Dweck MR. Pathophysiology of Aortic Stenosis and Future Perspectives for Medical Therapy. Cardiol Clin. 2020;38:1–12. [DOI] [PubMed] [Google Scholar]
- 26.Aggarwal SR, Clavel MA, Messika-Zeitoun D, Cueff C, Malouf J, Araoz PA, Mankad R, Michelena H, Vahanian A and Enriquez-Sarano M. Sex differences in aortic valve calcification measured by multidetector computed tomography in aortic stenosis. Circ Cardiovasc Imaging. 2013;6:40–7. [DOI] [PubMed] [Google Scholar]
- 27.Thaden JJ, Nkomo VT, Suri RM, Maleszewski JJ, Soderberg DJ, Clavel MA, Pislaru SV, Malouf JF, Foley TA, Oh JK, et al. Sex-related differences in calcific aortic stenosis: correlating clinical and echocardiographic characteristics and computed tomography aortic valve calcium score to excised aortic valve weight. Eur Heart J. 2016;37:693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clavel MA, Messika-Zeitoun D, Pibarot P, Aggarwal S, Malouf J, Araoz P, Michelena H, Cueff C, Larose É, Capoulade R, et al. The complex nature of discordant severe calcified aortic valve disease grading: New insights from combined Doppler-echocardiographic and computed tomographic study. J Am Coll Cardiol. 2013;62:2329–2338. [DOI] [PubMed] [Google Scholar]
- 29.Saeed S, Dweck MR and Chambers J. Sex differences in aortic stenosis: from pathophysiology to treatment. Expert Rev Cardiovasc Ther. 2020;18:65–76. [DOI] [PubMed] [Google Scholar]
- 30.McRobb L, Handelsman DJ and Heather AK. Androgen-induced progression of arterial calcification in apolipoprotein E-null mice is uncoupled from plaque growth and lipid levels. Endocrinology. 2009;150:841–8. [DOI] [PubMed] [Google Scholar]
- 31.Simard L, Côté N, Dagenais F, Mathieu P, Couture C, Trahan S, Bossé Y, Mohammadi S, Pagé S, Joubert P, et al. Sex-Related Discordance Between Aortic Valve Calcification and Hemodynamic Severity of Aortic Stenosis: Is Valvular Fibrosis the Explanation? Circ Res. 2017;120:681–691. [DOI] [PubMed] [Google Scholar]
- 32.Chaker Z, Badhwar V, Alqahtani F, Aljohani S, Zack CJ, Holmes DR, Rihal CS and Alkhouli M. Sex Differences in the Utilization and Outcomes of Surgical Aortic Valve Replacement for Severe Aortic Stenosis. J Am Heart Assoc. 2017;6:e006370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scully PR, Treibel TA, Fontana M, Lloyd G, Mullen M, Pugliese F, Hartman N, Hawkins PN, Menezes LJ and Moon JC. Prevalence of Cardiac Amyloidosis in Patients Referred for Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2018;71:463–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castaño A, Narotsky DL, Hamid N, Khalique OK, Morgenstern R, DeLuca A, Rubin J, Chiuzan C, Nazif T, Vahl T, et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38:2879–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kroi F, Fischer N, Gezin A, Hashim M and Rozenbaum MH. Estimating the Gender Distribution of Patients with Wild-Type Transthyretin Amyloid Cardiomyopathy: A Systematic Review and Meta-Analysis. Cardiol Ther. 2021;10:41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuchs C, Mascherbauer J, Rosenhek R, Pernicka E, Klaar U, Scholten C, Heger M, Wollenek G, Czerny M, Maurer G, et al. Gender differences in clinical presentation and surgical outcome of aortic stenosis. Heart. 2010;96:539–45. [DOI] [PubMed] [Google Scholar]
- 37.Klodas E, Enriquez-Sarano M, Tajik AJ, Mullany CJ, Bailey KR and Seward JB. Optimizing timing of surgical correction in patients with severe aortic regurgitation: role of symptoms. J Am Coll Cardiol. 1997;30:746–52. [DOI] [PubMed] [Google Scholar]
- 38.Kong WK, Regeer MV, Ng AC, McCormack L, Poh KK, Yeo TC, Shanks M, Parent S, Enache R, Popescu BA, et al. Sex Differences in Phenotypes of Bicuspid Aortic Valve and Aortopathy: Insights From a Large Multicenter, International Registry. Circ Cardiovasc Imaging. 2017;10:e005155. [DOI] [PubMed] [Google Scholar]
- 39.Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, Lancellotti P, LeFevre M, Miller F Jr. and Otto CM. Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30:372–392. [DOI] [PubMed] [Google Scholar]
- 40.Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:450–500. [DOI] [PubMed] [Google Scholar]
- 41.Zhu D, Hadoke PW, Wu J, Vesey AT, Lerman DA, Dweck MR, Newby DE, Smith LB and MacRae VE. Ablation of the androgen receptor from vascular smooth muscle cells demonstrates a role for testosterone in vascular calcification. Sci Rep. 2016;6:24807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomassen HK, Cioffi G, Gerdts E, Einarsen E, Midtbø HB, Mancusi C and Cramariuc D. Echocardiographic aortic valve calcification and outcomes in women and men with aortic stenosis. Heart. 2017;103:1619–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pawade T, Sheth T, Guzzetti E, Dweck MR and Clavel MA. Why and How to Measure Aortic Valve Calcification in Patients With Aortic Stenosis. JACC Cardiovasc Imaging. 2019;12:1835–1848. [DOI] [PubMed] [Google Scholar]
- 44.Voisine M, Hervault M, Shen M, Boilard AJ, Filion B, Rosa M, Bossé Y, Mathieu P, Côté N and Clavel MA. Age, Sex, and Valve Phenotype Differences in Fibro-Calcific Remodeling of Calcified Aortic Valve. J Am Heart Assoc. 2020;9:e015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clavel MA, Pibarot P, Messika-Zeitoun D, Capoulade R, Malouf J, Aggarval S, Araoz PA, Michelena HI, Cueff C, Larose E, et al. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: results of an international registry study. J Am Coll Cardiol. 2014;64:1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen V, Mathieu T, Melissopoulou M, Cimadevilla C, Codogno I, Huart V, Duval X, Vahanian A and Messika-Zeitoun D. Sex Differences in the Progression of Aortic Stenosis and Prognostic Implication: The COFRASA-GENERAC Study. JACC Cardiovasc Imaging. 2016;9:499–501. [DOI] [PubMed] [Google Scholar]
- 47.Tastet L, Kwiecinski J, Pibarot P, Capoulade R, Everett RJ, Newby DE, Shen M, Guzzetti E, Arsenault M, Bédard E, et al. Sex-related differences in the extent of myocardial fibrosis in patients with aortic valve stenosis. JACC Cardiovasc Imaging. 2020;13:699–711. [DOI] [PubMed] [Google Scholar]
- 48.Pighi M, Piazza N, Martucci G, Lachapelle K, Perrault LP, Asgar AW, Lauck S, Webb JG, Popma JJ, Kim DH, et al. Sex-Specific Determinants of Outcomes After Transcatheter Aortic Valve Replacement. Circ Cardiovasc Qual Outcomes. 2019;12:e005363. [DOI] [PubMed] [Google Scholar]
- 49.Capoulade R, Clavel MA, Le Ven F, Dahou A, Thébault C, Tastet L, Shen M, Arsenault M, Bédard É, Beaudoin J, et al. Impact of left ventricular remodelling patterns on outcomes in patients with aortic stenosis. Eur Heart J Cardiovasc Imaging. 2017;18:1378–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guzzetti E, Poulin A, Annabi MS, Zhang B, Kalavrouziotis D, Couture C, Dagenais F, Pibarot P and Clavel MA. Transvalvular Flow, Sex, and Survival After Valve Replacement Surgery in Patients With Severe Aortic Stenosis. J Am Coll Cardiol. 2020;75:1897–1909. [DOI] [PubMed] [Google Scholar]
- 51.Chandrasekhar J, Dangas G, Yu J, Vemulapalli S, Suchindran S, Vora AN, Baber U, Mehran R and Registry SAT. Sex-Based Differences in Outcomes With Transcatheter Aortic Valve Therapy: TVT Registry From 2011 to 2014. J Am Coll Cardiol. 2016;68:2733–2744. [DOI] [PubMed] [Google Scholar]
- 52.O’Connor SA, Morice MC, Gilard M, Leon MB, Webb JG, Dvir D, Rodes-Cabau J, Tamburino C, Capodanno D, D’Ascenzo F, et al. Revisiting Sex Equality With Transcatheter Aortic Valve Replacement Outcomes: A Collaborative, Patient-Level Meta-Analysis of 11,310 Patients. J Am Coll Cardiol. 2015;66:221–228. [DOI] [PubMed] [Google Scholar]
- 53.Vlastra W, Chandrasekhar J, Garcia Del Blanco B, Tchetche D, de Brito FS Jr., Barbanti M, Kornowski R, Latib A, D’Onofrio A, Ribichini F, et al. Sex differences in transfemoral transcatheter aortic valve replacement. J Am Coll Cardiol. 2019;74:2758–2767. [DOI] [PubMed] [Google Scholar]
- 54.Naoum C, Blanke P, Dvir D, Pibarot P, Humphries K, Webb J and Leipsic J. Clinical Outcomes and Imaging Findings in Women Undergoing TAVR. JACC Cardiovasc Imaging. 2016;9:483–93. [DOI] [PubMed] [Google Scholar]
- 55.Saad M, Nairooz R, Pothineni NVK, Almomani A, Kovelamudi S, Sardar P, Katz M, Abdel-Wahab M, Bangalore S, Kleiman NS, et al. Long-Term Outcomes With Transcatheter Aortic Valve Replacement in Women Compared With Men: Evidence From a Meta-Analysis. JACC Cardiovasc Interv. 2018;11:24–35. [DOI] [PubMed] [Google Scholar]
- 56.Szerlip M, Gualano S, Holper E, Squiers JJ, White JM, Doshi D, Williams MR, Hahn RT, Webb JG, Svensson LG, et al. Sex-Specific Outcomes of Transcatheter Aortic Valve Replacement With the SAPIEN 3 Valve: Insights From the PARTNER II S3 High-Risk and Intermediate-Risk Cohorts. JACC Cardiovasc Interv. 2018;11:13–20. [DOI] [PubMed] [Google Scholar]
- 57.Van Mieghem NM, Reardon MJ, Yakubov SJ, Heiser J, Merhi W, Windecker S, Makkar RR, Cheng W, Robbins M, Fail P, et al. Clinical outcomes of TAVI or SAVR in men and women with aortic stenosis at intermediate operative risk: a post hoc analysis of the randomised SURTAVI trial. EuroIntervention. 2020;16:833–841. [DOI] [PubMed] [Google Scholar]
- 58.Chiam PTL, Hayashida K, Watanabe Y, Yin WH, Kao HL, Lee MKY, Posas FE, Chandavimol M, Buddhari W, Dy TC, et al. Sex differences in patients undergoing transcatheter aortic valve replacement in Asia. Open Heart. 2021;8:e001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arnold SV, Spertus JA, Vemulapalli S, Li Z, Matsouaka RA, Baron SJ, Vora AN, Mack MJ, Reynolds MR, Rumsfeld JS, et al. Quality-of-Life Outcomes After Transcatheter Aortic Valve Replacement in an Unselected Population: A Report From the STS/ACC Transcatheter Valve Therapy Registry. JAMA Cardiol. 2017;2:409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lindman BR, Zajarias A, Maniar HS, Miller DC, Suri RM, Arnold SV, Webb J, Svensson LG, Kodali S, Xu K, et al. Risk stratification in patients with pulmonary hypertension undergoing transcatheter aortic valve replacement. Heart. 2015;101:1656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morris H, Denver N, Gaw R, Labazi H, Mair K and MacLean MR. Sex Differences in Pulmonary Hypertension. Clin Chest Med. 2021;42:217–228. [DOI] [PubMed] [Google Scholar]
- 62.Thenappan T, Shah SJ, Gomberg-Maitland M, Collander B, Vallakati A, Shroff P and Rich S. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2011;4:257–65. [DOI] [PubMed] [Google Scholar]
- 63.Pajjuru VS, Thandra A, Guddeti RR, Walters RW, Jhand A, Andukuri VG, Alkhouli M, Spertus JA and Md VMA. Sex Differences in Mortality and 90-day Readmission Rates after Transcatheter aortic valve replacement (TAVR): A Nationwide Analysis from the United States. Eur Heart J Qual Care Clin Outcomes. 2021:In press. [DOI] [PubMed] [Google Scholar]
- 64.Lowenstern A, Sheridan P, Wang TY, Boero I, Vemulapalli S, Thourani VH, Leon MB, Peterson ED and Brennan JM. Sex disparities in patients with symptomatic severe aortic stenosis. Am Heart J. 2021;237:116–126. [DOI] [PubMed] [Google Scholar]
- 65.Bienjonetti-Boudreau D, Fleury MA, Voisine M, Paquin A, Chouinard I, Tailleur M, Duval R, Magnan PO, Beaudoin J, Salaun E, et al. Impact of sex on the management and outcome of aortic stenosis patients. Eur Heart J. 2021;42:2683–2691. [DOI] [PubMed] [Google Scholar]
- 66.Onorati F, D’Errigo P, Barbanti M, Rosato S, Covello RD, Maraschini A, Ranucci M, Santoro G, Tamburino C, Grossi C, et al. Different impact of sex on baseline characteristics and major periprocedural outcomes of transcatheter and surgical aortic valve interventions: Results of the multicenter Italian OBSERVANT Registry. J Thorac Cardiovasc Surg. 2014;147:1529–39. [DOI] [PubMed] [Google Scholar]
- 67.Ryan CT, Almousa A, Zea-Vera R, Chang MQ, Amos CI, Coselli JS, Rosengart TK and Ghanta RK. Outcomes of Aortic Valve Replacement for Chronic Aortic Insufficiency: Analysis of the STS Database. Ann Thorac Surg. 2021:In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hernandez-Vaquero D, Rodriguez-Caulo E, Vigil-Escalera C, Blanco-Herrera O, Berastegui E, Arias-Dachary J, Souaf S, Parody G, Laguna G, Adsuar A, et al. Differences in life expectancy between men and women after aortic valve replacement. Eur J Cardiothorac Surg. 2021;60:681–688. [DOI] [PubMed] [Google Scholar]
- 69.Chieffo A, Petronio AS, Mehilli J, Chandrasekhar J, Sartori S, Lefèvre T, Presbitero P, Capranzano P, Tchetche D, Iadanza A, et al. 1-Year Clinical Outcomes in Women After Transcatheter Aortic Valve Replacement: Results From the First WIN-TAVI Registry. JACC Cardiovasc Interv. 2018;11:1–12. [DOI] [PubMed] [Google Scholar]
- 70.O’Brien SM, Feng L, He X, Xian Y, Jacobs JP, Badhwar V, Kurlansky PA, Furnary AP, Cleveland JC Jr., Lobdell KW, et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 2-Statistical Methods and Results. Ann Thorac Surg. 2018;105:1419–1428. [DOI] [PubMed] [Google Scholar]
- 71.Romano MA, Koeckert M, Mumtaz MA, Slachman FN, Patel HJ, Chitwood WR Jr., Barnhart GR, Grossi EA and Investigators TT. Permanent Pacemaker Implantation After Rapid Deployment Aortic Valve Replacement. Ann Thorac Surg. 2018;106:685–690. [DOI] [PubMed] [Google Scholar]
- 72.Bartko PE, Clavel MA, Annabi MS, Dahou A, Ristl R, Goliasch G, Baumgartner H, Hengstenberg C, Cavalcante JL, Burwash I, et al. Sex-Related Differences in Low-Gradient, Low-Ejection Fraction Aortic Stenosis: Results From the Multicenter TOPAS Study. JACC Cardiovasc Imaging. 2019;12:203–205. [DOI] [PubMed] [Google Scholar]
- 73.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, De Bonis M, Iung B, Johnson MR, Kintscher U, Kranke P, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3165–3241. [DOI] [PubMed] [Google Scholar]
- 74.Siu SC, Sermer M, Colman JM, Alvarez AN, Mercier LA, Morton BC, Kells CM, Bergin ML, Kiess MC, Marcotte F, et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 2001;104:515–21. [DOI] [PubMed] [Google Scholar]
- 75.Drenthen W, Boersma E, Balci A, Moons P, Roos-Hesselink JW, Mulder BJ, Vliegen HW, van Dijk AP, Voors AA, Yap SC, et al. Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J. 2010;31:2124–32. [DOI] [PubMed] [Google Scholar]
- 76.Egidy Assenza G, Dimopoulos K, Budts W, Donti A, Economy KE, Gargiulo GD, Gatzoulis M, Landzberg MJ, Valente AM and Roos-Hesselink J. Management of acute cardiovascular complications in pregnancy. Eur Heart J. 2021;42:4224–4240. [DOI] [PubMed] [Google Scholar]
- 77.Mehta LS, Warnes CA, Bradley E, Burton T, Economy K, Mehran R, Safdar B, Sharma G, Wood M, Valente AM, et al. Cardiovascular Considerations in Caring for Pregnant Patients: A Scientific Statement From the American Heart Association. Circulation. 2020;141:e884–e903. [DOI] [PubMed] [Google Scholar]
- 78.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG and Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–11. [DOI] [PubMed] [Google Scholar]
- 79.Yadgir S, Johnson CO, Aboyans V, Adebayo OM, Adedoyin RA, Afarideh M, Alahdab F, Alashi A, Alipour V, Arabloo J, et al. Global, Regional, and National Burden of Calcific Aortic Valve and Degenerative Mitral Valve Diseases, 1990–2017. Circulation. 2020;141:1670–1680. [DOI] [PubMed] [Google Scholar]
- 80.Vakamudi S, Jellis C, Mick S, Wu Y, Gillinov AM, Mihaljevic T, Cosgrove DM, Svensson L and Cho L. Sex Differences in the Etiology of Surgical Mitral Valve Disease. Circulation. 2018;138:1749–1751. [DOI] [PubMed] [Google Scholar]
- 81.Delling FN and Vasan RS. Epidemiology and pathophysiology of mitral valve prolapse: new insights into disease progression, genetics, and molecular basis. Circulation. 2014;129:2158–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, Lehman B and Benjamin EJ. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999;341:1–7. [DOI] [PubMed] [Google Scholar]
- 83.Devereux RB, Brown WT, Kramer-Fox R and Sachs I. Inheritance of mitral valve prolapse: effect of age and sex on gene expression. Ann Intern Med. 1982;97:826–32. [DOI] [PubMed] [Google Scholar]
- 84.Le Tourneau T, Le Scouarnec S, Cueff C, Bernstein D, Aalberts JJJ, Lecointe S, Mérot J, Bernstein JA, Oomen T, Dina C, et al. New insights into mitral valve dystrophy: a Filamin-A genotype-phenotype and outcome study. Eur Heart J. 2018;39:1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Toomer KA, Yu M, Fulmer D, Guo L, Moore KS, Moore R, Drayton KD, Glover J, Peterson N, Ramos-Ortiz S, et al. Primary cilia defects causing mitral valve prolapse. Sci Transl Med. 2019;11:eaax0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Durst R, Sauls K, Peal DS, deVlaming A, Toomer K, Leyne M, Salani M, Talkowski ME, Brand H, Perrocheau M, et al. Mutations in DCHS1 cause mitral valve prolapse. Nature. 2015;525:109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Avierinos JF, Inamo J, Grigioni F, Gersh B, Shub C and Enriquez-Sarano M. Sex differences in morphology and outcomes of mitral valve prolapse. Ann Intern Med. 2008;149:787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Elmariah S, Budoff MJ, Delaney JA, Hamirani Y, Eng J, Fuster V, Kronmal RA, Halperin JL and O’Brien KD. Risk factors associated with the incidence and progression of mitral annulus calcification: the multi-ethnic study of atherosclerosis. Am Heart J. 2013;166:904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seeburger J, Eifert S, Pfannmüller B, Garbade J, Vollroth M, Misfeld M, Borger M and Mohr FW. Gender differences in mitral valve surgery. Thorac Cardiovasc Surg. 2013;61:42–6. [DOI] [PubMed] [Google Scholar]
- 90.Giustino G, Overbey J, Taylor D, Ailawadi G, Kirkwood K, DeRose J, Gillinov MA, Dagenais F, Mayer ML, Moskowitz A, et al. Sex-Based Differences in Outcomes After Mitral Valve Surgery for Severe Ischemic Mitral Regurgitation: From the Cardiothoracic Surgical Trials Network. JACC Heart Fail. 2019;7:481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hart SA, Krasuski RA, Wang A, Kisslo K, Harrison JK and Bashore TM. Pulmonary hypertension and elevated transpulmonary gradient in patients with mitral stenosis. J Heart Valve Dis. 2010;19:708–15. [PubMed] [Google Scholar]
- 92.Ge Y, Gupta S, Fentanes E, Aghayev A, Steigner M, Sobieszczyk P, Kaneko T, Di Carli MF, Bhatt DL, Shah P, et al. Role of Cardiac CT in Pre-Procedure Planning for Transcatheter Mitral Valve Replacement. JACC Cardiovasc Imaging. 2021;14:1571–1580. [DOI] [PubMed] [Google Scholar]
- 93.Mantovani F, Clavel MA, Michelena HI, Suri RM, Schaff HV and Enriquez-Sarano M. Comprehensive Imaging in Women With Organic Mitral Regurgitation: Implications for Clinical Outcome. JACC Cardiovasc Imaging. 2016;9:388–96. [DOI] [PubMed] [Google Scholar]
- 94.McNeely C and Vassileva C. Mitral Valve Surgery in Women: Another Target for Eradicating Sex Inequality. Circ Cardiovasc Qual Outcomes. 2016;9:S94–6. [DOI] [PubMed] [Google Scholar]
- 95.Adamo M, Godino C, Giannini C, Scotti A, Liga R, Curello S, Fiorina C, Chiari E, Chizzola G, Abbenante A, et al. Left ventricular reverse remodelling predicts long-term outcomes in patients with functional mitral regurgitation undergoing MitraClip therapy: results from a multicentre registry. Eur J Heart Fail. 2019;21:196–204. [DOI] [PubMed] [Google Scholar]
- 96.Kosmidou I, Lindenfeld J, Abraham WT, Rinaldi MJ, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Gaba P, Rogers JH, et al. Sex-Specific Outcomes of Transcatheter Mitral-Valve Repair and Medical Therapy for Mitral Regurgitation in Heart Failure. JACC Heart Fail. 2021;9:674–683. [DOI] [PubMed] [Google Scholar]
- 97.Mutagaywa RK, Wind AM, Kamuhabwa A, Cramer MJ, Chillo P and Chamuleau S. Rheumatic heart disease anno 2020: Impacts of gender and migration on epidemiology and management. Eur J Clin Invest. 2020;50:e13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bonow RO, O’Gara PT, Adams DH, Badhwar V, Bavaria JE, Elmariah S, Hung JW, Lindenfeld J, Morris AA, Satpathy R, et al. 2020 Focused Update of the 2017 ACC Expert Consensus Decision Pathway on the Management of Mitral Regurgitation. J Am Coll Cardiol. 2020;75:2236–2270. [DOI] [PubMed] [Google Scholar]
- 99.Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–406. [DOI] [PubMed] [Google Scholar]
- 100.Attizzani GF, Ohno Y, Capodanno D, Cannata S, Dipasqua F, Imme S, Mangiafico S, Barbanti M, Ministeri M, Cageggi A, et al. Extended use of percutaneous edge-to-edge mitral valve repair beyond EVEREST (Endovascular Valve Edge-to-Edge Repair) criteria: 30-day and 12-month clinical and echocardiographic outcomes from the GRASP (Getting Reduction of Mitral Insufficiency by Percutaneous Clip Implantation) registry. JACC Cardiovasc Interv. 2015;8:74–82. [DOI] [PubMed] [Google Scholar]
- 101.Bottari VE, Tamborini G, Bartorelli AL, Alamanni F and Pepi M. MitraClip implantation in a previous surgical mitral valve edge-to-edge repair. JACC Cardiovasc Interv. 2015;8:111–3. [DOI] [PubMed] [Google Scholar]
- 102.Estevez-Loureiro R, Franzen O, Winter R, Sondergaard L, Jacobsen P, Cheung G, Moat N, Ihlemann N, Ghione M, Price S, et al. Echocardiographic and clinical outcomes of central versus noncentral percutaneous edge-to-edge repair of degenerative mitral regurgitation. J Am Coll Cardiol. 2013;62:2370–7. [DOI] [PubMed] [Google Scholar]
- 103.Hahn RT. Transcathether Valve Replacement and Valve Repair: Review of Procedures and Intraprocedural Echocardiographic Imaging. Circ Res. 2016;119:341–56. [DOI] [PubMed] [Google Scholar]
- 104.Basso C, Iliceto S, Thiene G and Perazzolo Marra M. Mitral Valve Prolapse, Ventricular Arrhythmias, and Sudden Death. Circulation. 2019;140:952–964. [DOI] [PubMed] [Google Scholar]
- 105.Guerrero M, Urena M, Himbert D, Wang DD, Eleid M, Kodali S, George I, Chakravarty T, Mathur M, Holzhey D, et al. 1-Year Outcomes of Transcatheter Mitral Valve Replacement in Patients With Severe Mitral Annular Calcification. J Am Coll Cardiol. 2018;71:1841–1853. [DOI] [PubMed] [Google Scholar]
- 106.Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N Engl J Med. 2018;379:2307–2318. [DOI] [PubMed] [Google Scholar]
- 107.Obadia JF, Messika-Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, Lefevre T, Piot C, Rouleau F, Carrie D, et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N Engl J Med. 2018;379:2297–2306. [DOI] [PubMed] [Google Scholar]
- 108.Werner N, Puls M, Baldus S, Lubos E, Bekeredjian R, Sievert H, Schofer J, Kuck KH, Mollmann H, Hehrlein C, et al. Gender-related differences in patients undergoing transcatheter mitral valve interventions in clinical practice: 1-year results from the German TRAMI registry. Catheter Cardiovasc Interv. 2020;95:819–829. [DOI] [PubMed] [Google Scholar]
- 109.Attizzani GF, Ohno Y, Capodanno D, Cannata S, Dipasqua F, Immé S, Mangiafico S, Barbanti M, Ministeri M, Cageggi A, et al. Gender-related clinical and echocardiographic outcomes at 30-day and 12-month follow up after MitraClip implantation in the GRASP registry. Catheter Cardiovasc Interv. 2015;85:889–97. [DOI] [PubMed] [Google Scholar]
- 110.Park SD, Orban M, Karam N, Lubos E, Kalbacher D, Braun D, Stolz L, Neuss M, Butter C, Praz F, et al. Sex-Related Clinical Characteristics and Outcomes of Patients Undergoing Transcatheter Edge-to-Edge Repair for Secondary Mitral Regurgitation. JACC Cardiovasc Interv. 2021;14:819–827. [DOI] [PubMed] [Google Scholar]
- 111.Messika-Zeitoun D, Candolfi P, Vahanian A, Chan V, Burwash IG, Philippon JF, Toussaint JM, Verta P, Feldman TE, Iung B, et al. Dismal Outcomes and High Societal Burden of Mitral Valve Regurgitation in France in the Recent Era: A Nationwide Perspective. J Am Heart Assoc. 2020;9:e016086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vassileva CM, McNeely C, Mishkel G, Boley T, Markwell S and Hazelrigg S. Gender differences in long-term survival of Medicare beneficiaries undergoing mitral valve operations. Ann Thorac Surg. 2013;96:1367–1373. [DOI] [PubMed] [Google Scholar]
- 113.Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72–e227. [DOI] [PubMed] [Google Scholar]
- 114.Messika-Zeitoun D, Candolfi P, Enriquez-Sarano M, Burwash IG, Chan V, Philippon JF, Toussaint JM, Verta P, Feldman TE, Iung B, et al. Presentation and outcomes of mitral valve surgery in France in the recent era: a nationwide perspective. Open Heart. 2020;7:e001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Safi LM and Tsiaras SV. Update on Valvular Heart Disease in Pregnancy. Curr Treat Options Cardiovasc Med. 2017;19:70. [DOI] [PubMed] [Google Scholar]
- 116.de Souza JA, Martinez EE Jr., Ambrose JA, Alves CM, Born D, Buffolo E and Carvalho AC. Percutaneous balloon mitral valvuloplasty in comparison with open mitral valve commissurotomy for mitral stenosis during pregnancy. J Am Coll Cardiol. 2001;37:900–3. [DOI] [PubMed] [Google Scholar]
- 117.Dahou A, Levin D, Reisman M and Hahn RT. Anatomy and Physiology of the Tricuspid Valve. JACC Cardiovasc Imaging. 2019;12:458–468. [DOI] [PubMed] [Google Scholar]
- 118.Topilsky Y, Maltais S, Medina Inojosa J, Oguz D, Michelena H, Maalouf J, Mahoney DW and Enriquez-Sarano M. Burden of Tricuspid Regurgitation in Patients Diagnosed in the Community Setting. JACC Cardiovasc Imaging. 2019;12:433–442. [DOI] [PubMed] [Google Scholar]
- 119.Gupta A, Grover V and Gupta VK. Congenital tricuspid regurgitation: review and a proposed new classification. Cardiol Young. 2011;21:121–9. [DOI] [PubMed] [Google Scholar]
- 120.Møller JE, Pellikka PA, Bernheim AM, Schaff HV, Rubin J and Connolly HM. Prognosis of carcinoid heart disease: analysis of 200 cases over two decades. Circulation. 2005;112:3320–7. [DOI] [PubMed] [Google Scholar]
- 121.Hahn RT, Weckbach LT, Noack T, Hamid N, Kitamura M, Bae R, Lurz P, Kodali SK, Sorajja P, Hausleiter J, et al. Proposal for a Standard Echocardiographic Tricuspid Valve Nomenclature. JACC Cardiovasc Imaging. 2021;14:1299–1305. [DOI] [PubMed] [Google Scholar]
- 122.Prihadi EA, van der Bijl P, Gursoy E, Abou R, Mara Vollema E, Hahn RT, Stone GW, Leon MB, Ajmone Marsan N, Delgado V, et al. Development of significant tricuspid regurgitation over time and prognostic implications: new insights into natural history. Eur Heart J. 2018;39:3574–3581. [DOI] [PubMed] [Google Scholar]
- 123.El-Busaid H, Hassan S, Odula P, Ogeng’o J and Ndung’u B. Sex variations in the structure of human atrioventricular annuli. Folia Morphol (Warsz). 2012;71:23–7. [PubMed] [Google Scholar]
- 124.Dietz MF, Prihadi EA, van der Bijl P, Fortuni F, Marques AI, Ajmone Marsan N, Bax JJ and Delgado V. Sex-Specific Differences in Etiology and Prognosis in Patients With Significant Tricuspid Regurgitation. Am J Cardiol. 2021;147:109–115. [DOI] [PubMed] [Google Scholar]
- 125.Gual-Capllonch F, Cediel G, Ferrer E, Teis A, Juncà G, Vallejo N, López-Ayerbe J and Bayes-Genis A. Sex-Related Differences in the Mechanism of Functional Tricuspid Regurgitation. Heart Lung Circ. 2021;30:e16–e22. [DOI] [PubMed] [Google Scholar]
- 126.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK and Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713; quiz 786–8. [DOI] [PubMed] [Google Scholar]
- 127.Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303–371. [DOI] [PubMed] [Google Scholar]
- 128.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 129.Lang RM, Badano LP, Tsang W, Adams DH, Agricola E, Buck T, Faletra FF, Franke A, Hung J, de Isla LP, et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr. 2012;25:3–46. [DOI] [PubMed] [Google Scholar]
- 130.Badano LP, Agricola E, de Isla LP, Gianfagna P and Zamorano JL. Evaluation of the tricuspid valve morphology and function by transthoracic real-time three-dimensional echocardiography. Eur J Echocardiogr. 2009;10:477–484. [DOI] [PubMed] [Google Scholar]
- 131.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. [DOI] [PubMed] [Google Scholar]
- 132.Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C, Hagendorff A, Monin JL, Badano L and Zamorano JL. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr. 2010;11:307–32. [DOI] [PubMed] [Google Scholar]
- 133.Dahou A, Ong G, Hamid N, Avenatti E, Yao J and Hahn RT. Quantifying Tricuspid Regurgitation Severity: A Comparison of Proximal Isovelocity Surface Area and Novel Quantitative Doppler Methods. JACC Cardiovasc Imaging. 2019;12:560–562. [DOI] [PubMed] [Google Scholar]
- 134.Hahn RT. State-of-the-Art Review of Echocardiographic Imaging in the Evaluation and Treatment of Functional Tricuspid Regurgitation. Circ Cardiovasc Imaging. 2016;9:e005332. [DOI] [PubMed] [Google Scholar]
- 135.Hahn RT and Zamorano JL. The need for a new tricuspid regurgitation grading scheme. Eur Heart J Cardiovasc Imaging. 2017;18:1342–1343. [DOI] [PubMed] [Google Scholar]
- 136.Santoro C, Marco Del Castillo A, Gonzalez-Gomez A, Monteagudo JM, Hinojar R, Lorente A, Abellas M, Vieitez JM, Garcia Martin A, Casas Rojo E, et al. Mid-term outcome of severe tricuspid regurgitation: are there any differences according to mechanism and severity? Eur Heart J Cardiovasc Imaging. 2019;20:1035–1042. [DOI] [PubMed] [Google Scholar]
- 137.Peri Y, Sadeh B, Sherez C, Hochstadt A, Biner S, Aviram G, Ingbir M, Nachmany I, Topaz G, Flint N, et al. Quantitative assessment of effective regurgitant orifice: impact on risk stratification, and cut-off for severe and torrential tricuspid regurgitation grade. Eur Heart J Cardiovasc Imaging. 2019;21:768–776. [DOI] [PubMed] [Google Scholar]
- 138.Utsunomiya H, Harada Y, Susawa H, Takahari K, Ueda Y, Izumi K, Itakura K, Ikenaga H, Hidaka T, Fukuda Y, et al. Comprehensive Evaluation of Tricuspid Regurgitation Location and Severity Using Vena Contracta Analysis: A Color Doppler Three-Dimensional Transesophageal Echocardiographic Study. J Am Soc Echocardiogr. 2019;32:1526–1537.e2. [DOI] [PubMed] [Google Scholar]
- 139.Kebed KY, Addetia K, Henry M, Yamat M, Weinert L, Besser SA, Mor-Avi V and Lang RM. Refining Severe Tricuspid Regurgitation Definition by Echocardiography with a New Outcomes-Based “Massive” Grade. J Am Soc Echocardiogr. 2020;33:1087–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2021:In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hahn RT, Thomas JD, Khalique OK, Cavalcante JL, Praz F and Zoghbi WA. Imaging Assessment of Tricuspid Regurgitation Severity. JACC Cardiovasc Imaging. 2019;12:469–490. [DOI] [PubMed] [Google Scholar]
- 142.Khalique OK, Cavalcante JL, Shah D, Guta AC, Zhan Y, Piazza N and Muraru D. Multimodality Imaging of the Tricuspid Valve and Right Heart Anatomy. JACC Cardiovasc Imaging. 2019;12:516–531. [DOI] [PubMed] [Google Scholar]
- 143.Petersen SE, Khanji MY, Plein S, Lancellotti P and Bucciarelli-Ducci C. European Association of Cardiovascular Imaging expert consensus paper: a comprehensive review of cardiovascular magnetic resonance normal values of cardiac chamber size and aortic root in adults and recommendations for grading severity. Eur Heart J Cardiovasc Imaging. 2019;20:1321–1331. [DOI] [PubMed] [Google Scholar]
- 144.Hahn RT, Kodali S, Fam N, Bapat V, Bartus K, Rodés-Cabau J, Dagenais F, Estevez-Loureiro R, Forteza A, Kapadia S, et al. Early Multinational Experience of Transcatheter Tricuspid Valve Replacement for Treating Severe Tricuspid Regurgitation. JACC Cardiovasc Interv. 2020;13:2482–2493. [DOI] [PubMed] [Google Scholar]
- 145.Nickenig G, Weber M, Lurz P, von Bardeleben RS, Sitges M, Sorajja P, Hausleiter J, Denti P, Trochu JN, Nabauer M, et al. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet. 2019;394:2002–2011. [DOI] [PubMed] [Google Scholar]
- 146.Kodali S, Hahn RT, Eleid MF, Kipperman R, Smith R, Lim DS, Gray WA, Narang A, Pislaru SV, Koulogiannis K, et al. Feasibility Study of the Transcatheter Valve Repair System for Severe Tricuspid Regurgitation. J Am Coll Cardiol. 2021;77:345–356. [DOI] [PubMed] [Google Scholar]
- 147.Fender EA, Petrescu I, Ionescu F, Zack CJ, Pislaru SV, Nkomo VT, Cochuyt JJ, Hodge DO and Nishimura RA. Prognostic Importance and Predictors of Survival in Isolated Tricuspid Regurgitation: A Growing Problem. Mayo Clin Proc. 2019;94:2032–2039. [DOI] [PubMed] [Google Scholar]
- 148.Zack CJ, Fender EA, Chandrashekar P, Reddy YNV, Bennett CE, Stulak JM, Miller VM and Nishimura RA. National Trends and Outcomes in Isolated Tricuspid Valve Surgery. J Am Coll Cardiol. 2017;70:2953–2960. [DOI] [PubMed] [Google Scholar]
- 149.Colombo A and Maisano F. A new tool for the forgotten valve: a score to predict the risk of surgery. Eur Heart J. 2021:In press. [DOI] [PubMed] [Google Scholar]
- 150.Dreyfus J, Audureau E, Bohbot Y, Coisne A, Lavie-Badie Y, Bouchery M, Flagiello M, Bazire B, Eggenspieler F, Viau F, et al. TRI-SCORE: a new risk score for in-hospital mortality prediction after isolated tricuspid valve surgery. Eur Heart J. 2021:In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Aggarwal V, Mulukutla V, Maskatia S, Justino H, Mullins CE and Qureshi AM. Outcomes after Balloon Pulmonary Valvuloplasty for Critical Pulmonary Stenosis and Incidence of Coronary Artery Fistulas. Am J Cardiol. 2018;121:1617–1623. [DOI] [PubMed] [Google Scholar]
- 152.LaHaye S, Lincoln J and Garg V. Genetics of valvular heart disease. Curr Cardiol Rep. 2014;16:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ruckdeschel E and Kim YY. Pulmonary valve stenosis in the adult patient: pathophysiology, diagnosis and management. Heart. 2019;105:414–422. [DOI] [PubMed] [Google Scholar]
- 154.McCrindle BW. Independent predictors of long-term results after balloon pulmonary valvuloplasty. Valvuloplasty and Angioplasty of Congenital Anomalies (VACA) Registry Investigators. Circulation. 1994;89:1751–9. [DOI] [PubMed] [Google Scholar]
- 155.Ananthakrishna A, Balasubramonium VR, Thazhath HK, Saktheeshwaran M, Selvaraj R, Satheesh S and Jayaraman B. Balloon pulmonary valvuloplasty in adults: immediate and long-term outcomes. J Heart Valve Dis. 2014;23:511–5. [PubMed] [Google Scholar]
- 156.Hameed AB, Goodwin TM and Elkayam U. Effect of pulmonary stenosis on pregnancy outcomes--a case-control study. Am Heart J. 2007;154:852–4. [DOI] [PubMed] [Google Scholar]
- 157.Drenthen W, Pieper PG, Roos-Hesselink JW, Schmidt AC, Mulder BJ, van Dijk AP, Vliegen HW, Sollie KM, Voors AA, Ebels T, et al. Non-cardiac complications during pregnancy in women with isolated congenital pulmonary valvar stenosis. Heart. 2006;92:1838–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lindley K, Williams D. Valvular Heart Disease in Pregnancy. American College of Cardiology. 2018. Accessed October 3, 2021. https://www.acc.org/latest-in-cardiology/articles/2018/02/12/07/29/http%3a%2f%2fwww.acc.org%2flatest-in-cardiology%2farticles%2f2018%2f02%2f12%2f07%2f29%2fvalvular-heart-disease-in-pregnancy