Abstract
AXL and its corresponding ligand growth arrest–specific 6 (GAS‐6) are critically involved in hepatic immunomodulation and regenerative processes. Pleiotropic inhibitory effects on innate inflammatory responses might essentially involve the shift of macrophage phenotype from a pro‐inflammatory M1 to an anti‐inflammatory M2. We aimed to assess the relevance of the AXL/GAS‐6‐pathway in human liver regeneration and, consequently, its association with clinical outcome after hepatic resection. Soluble AXL (sAXL) and GAS‐6 levels were analyzed at preoperative and postoperative stages in 154 patients undergoing partial hepatectomy and correlated with clinical outcome. Perioperative dynamics of interleukin (IL)‐6, soluble tyrosine‐protein kinase MER (sMerTK), soluble CD163 (sCD163), and cytokeratin (CK) 18 were assessed to reflect pathophysiological processes. Preoperatively elevated sAXL and GAS‐6 levels predicted postoperative liver dysfunction (area under the curve = 0.721 and 0.722; P < 0.005) and worse clinical outcome. These patients failed to respond with an immediate increase of sAXL and GAS‐6 upon induction of liver regeneration. Abolished AXL pathway response resulted in a restricted increase of sCD163, suggesting a disrupted phenotypical switch to regeneratory M2 macrophages. No association with sMerTK was observed. Concomitantly, a distinct association of IL‐6 levels with an absent increase of AXL/GAS‐6 signaling indicated pronounced postoperative inflammation. This was further supported by increased intrahepatic secondary necrosis as reflected by CK18M65. sAXL and GAS‐6 represent not only potent and easily accessible preoperative biomarkers for the postoperative outcome but also AXL/GAS‐6 signaling might be of critical relevance in human liver regeneration. Refractory AXL/GAS‐6 signaling, due to chronic overactivation/stimulation in the context of underlying liver disease, appears to abolish their immediate release following induction of liver regeneration, causing overwhelming immune activation, presumably via intrahepatic immune regulation.

Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AUC
area under the curve
- CD163
cluster of differentiation 163
- CLD
chronic liver disease
- CK18
cytokeratin 18
- cCK18
caspase‐cleaved cytokeratin 18
- GAS‐6
growth arrest–specific 6
- GGT
gamma‐glutamyl transferase
- ICU
intensive care unit
- IL
interleukin
- IFN
interferon
- LD
liver dysfunction
- POD
postoperative days
- PREOP
preoperative
- ROC
receiver operating characteristic
- sAXL
soluble Axl
- sMerTK
soluble tyrosine‐protein kinase MER
- SOCS1/3
suppressor of cytokine signaling 1/3
- TAM
acronym Tyro3, Axl and MerTK receptor tyrosine kinases
- TGF‐β
transforming growth factor beta
- TNF‐α
tumor necrosis factor alpha
The human liver is an organ with remarkable regenerative capacity. Nevertheless, specifically in the context of preexisting liver disease, liver resection might exceed the regenerative reserve. Accordingly, the extent of resection and preexisting functional hepatic impairment have to be carefully assessed, as both affect postoperative recovery.( 1 , 2 ) Especially in humans, in whom underlying liver disease might further affect regenerative processes, liver regeneration is still only incompletely understood.( 3 ) Postoperative liver dysfunction (LD), as clinical correlate of impaired regeneration, remains a frequently observed complication of resection as well as a primary determinant of postoperative mortality.( 4 ) As there are currently only limited treatment options for patients who develop postoperative LD, prevention via the estimation of patients’ individual risk and subsequent adaptation of surgical strategy are of utmost importance.
Liver regeneration relies on a well‐coordinated interplay of various components that might be disrupted in patients with underlying liver disease. In healthy livers, macrophages were shown to be of importance in a precisely regulated inflammatory response.( 3 , 5 ) Accordingly, macrophage dysfunction could be noted in a variety of chronic liver diseases (CLDs).( 6 ) Also, in the context of liver regeneration, macrophages are of distinct relevance, as the depletion of Kupffer cells is associated with delayed liver regeneration in mice.( 7 , 8 ) As resident macrophages, they not only immediately release cytokines relevant for liver regeneration (e.g., interleukin 6 [IL‐6], tumor necrosis factor alpha [TNF‐α]) but were shown to be a guarantee of an orderly inflammatory process.( 9 )
However, Kupffer cells exhibit tremendous plasticity, depending on the local metabolic and immune environment. A shift in macrophage polarization, from a pro‐inflammatory M1 to a pro‐regeneratory M2 phenotype, has been well established.( 10 ) These phenotypical changes appear to be essential during regenerative processes. In particular, immediately following tissue injury, pro‐inflammatory M1 macrophages are prevailing. Throughout regeneration, though, M1/M2 balance shifts, making pro‐regenerative M2 macrophages the predominant subtype.( 11 ) M2 polarization not only dampens pro‐inflammatory M1 responses but also promotes tissue repair.( 10 , 11 , 12 ) Indeed, missing intrahepatic M2 polarization has been associated with prolonged inflammation and reduced regeneration after liver injury.( 13 ) Although M1/M2 dichotomy provides a conceptual framework for our understanding of macrophages and their ambivalent role in the setting of injury, their way of orchestrating inflammation and its resolution is still incompletely understood and might be very dynamic, specifically in the setting of underlying liver disease.( 14 )
In this context, TAM receptors (Tyro3, Axl, and Mer receptor tyrosine kinases) and corresponding ligands (GAS‐6 [growth arrest–specific 6], protein S) have been shown to be critically involved in hepatic immunomodulation and further are up‐regulated in patients with liver disease.( 15 , 16 , 17 ) Accumulating evidence supports rather pleiotropic inhibitory effects on innate inflammatory responses.( 18 , 19 , 20 ) Interestingly, TAM receptors are also involved in macrophage polarization. Mediating the engulfment of apoptotic bodies, they promote macrophage M2 shift, a process critically disrupted in patients with CLD.( 17 , 21 , 22 ) In accordance, the hepatic immunological balance is disturbed in the absence of TAM signaling, as an excess of inflammatory cytokines indicated pronounced inflammation due to insufficient inhibition of innate inflammatory responses.( 23 , 24 ) In mice, deficient TAM signaling was linked to pronounced inflammation and delayed liver regeneration following acute liver injury.( 23 , 24 ) Furthermore, mice with a deficiency of either AXL or GAS‐6 suffered from impaired regeneration with a much higher incidence than wild‐type animals.( 23 , 24 )
Given the established association of TAM signaling and CLD, as well as its potential relevance in liver regeneration, we aimed to (1) explore whether preoperative soluble Axl (sAXL) and GAS‐6 levels could predict postoperative outcome after liver resection, (2) identify potential differences in their perioperative dynamics in patients with and without insufficient postoperative liver regeneration, and (3) gain mechanistic insight into how TAM signaling might affect liver regeneration in humans, particularly on the background of underlying liver disease.
Patients and Methods
For this study, a total of 154 patients were recruited at two different institutions, comprising the General Hospital of Vienna/Medical University of Vienna (Austria) and the hospital Klinikum Favoriten (Austria). Patients undergoing liver resection were followed prospectively over a postoperative period of 90 days. sAXL, GAS‐6, and soluble tyrosine‐protein kinase MER (sMerTK) as well as soluble cluster of differentiation 163 (sCD163), IL‐6, and cytokeratin 18 (CK18) were evaluated within the immediate perioperative period (1 day before surgery [PREOP] and 1 day [POD1] as well as 5 days afterward [POD5]. Furthermore, in a subset of 63 patients, intraoperative samples were obtained (see Supporting Methods) to evaluate alterations in the early phase of liver regeneration.
Essential patient‐related data were assessed as listed in Table 1. The extent of resection was classified as minor or major resection (< 3 segments = minor; ≥ 3 segments = major) according to the IHPBA‐Brisbane‐2000 nomenclature.( 25 ) Postoperative outcome was prospectively documented and classified in LD (International Study Group of Liver Surgery criteria), postoperative morbidity (Calvien‐Dindo classification), and postoperative mortality.( 26 , 27 ) For more details, refer to the Supporting Methods section.
TABLE 1.
Demographics of Entire Cohort
| Cohort Total (n = 154) | Cohort AXLlow (n = 70) | Cohort AXLhigh (n = 80) | Cohort GAS‐6low (n = 99) | Cohort GAS‐6high (n = 44) | |||
|---|---|---|---|---|---|---|---|
| n (%) – Median [Range] | |||||||
| Age (years) | 64.2 (22.16‐89.31) | 61.7 (31.7‐89.3) | 65.2 (22.2‐86.1) | [0.154] | 64.1 (22.16‐89.31) | 65.2 (35.61‐84.68) | [0.112] |
| Sex | |||||||
| Male | 105 (68.2) | 43 (61.4) | 60 (75.0) | [0.074] | 70 (70.7) | 32 (72.7) | [0.805] |
| Female | 49 (31.8) | 27 (38.6) | 20 (25.0) | [0.074] | 29 (29.3) | 12 (27.3) | [0.805] |
| Neoplastic entity | |||||||
| mCRC | 62 (40.3) | 49 (70.0) | 13 (16.2) | <0.005 | 57 (57.6) | 5 (11.4) | <0.005 |
| HCC | 50 (32.5) | 4 (5.7) | 44 (55.0) | <0.005 | 20 (20.2) | 28 (63.3) | <0.005 |
| CCC | 25 (16.2) | 7 (10.0) | 16 (20.0) | [0.09] | 12 (12.1) | 10 (22.7) | [0.105] |
| Non‐neoplastic | 12 (7.8) | 8 (11.4) | 4 (5.0) | [0.148] | 7 (7.1) | 0 (0.0) | [0.1] |
| Other | 5 (3.2) | 2 (2.9) | 3 (3.8) | [1.0] | 3 (3.0) | 1 (2.3) | [0.8] |
| Resection extent | |||||||
| Major | 98 (63.6) | 46 (65.7) | 49 (61.2) | [0.571] | 64 (64.6) | 28 (63.6) | [0.907] |
| Minor | 56 (36.4) | 24 (34.3) | 31 (38.8) | [0.571] | 35 (35.4) | 16 (36.4) | [0.907] |
| Outcome | |||||||
| Postoperative LD—yes | 18 (11.7) | 1 (1.7) | 15 (21.7) | <0.005 | 4 (4.8) | 12 (30.8) | <0.005 |
| Morbidity—yes | 77 (50.0) | 26 (37.1) | 47 (58.8) | [0.008] | 36 (36.4) | 33 (75.0) | <0.005 |
| Severe morbidity—yes | 40 (26.0) | 13 (18.6) | 25 (31.3) | [0.075] | 15 (15.2) | 21 (47.7) | <0.005 |
| 90‐day mortality—yes | 5 (3.2) | 0 (0.0) | 4 (5.3) | [0.123] | 0 (0.0) | 4 (9.8) | <0.005 |
| ICU days | 1.0 (0‐26) | 1.0 (0‐5) | 1.0 (0‐26) | [0.123] | 1.0 (0‐10) | 2.0 (0‐26) | [0.014] |
| Hospitalization days | 8.0 (3‐117) | 8.0 (4‐61) | 10.0 (3‐117) | [0.53] | 8.0 (3‐61) | 12.5 (4‐117) | <0.005 |
| Liver histology | |||||||
| Fibrosis—yes | 112 (72.7) | 46 (73.0) | 63 (81.8) | [0.212] | 71 (74.7) | 34 (82.9) | [0.296] |
| Grade 0‐II | 77 (75.6) | 57 (90.5) | 51 (66.2) | <0.005 | 82 (86.3) | 23 (56.1) | <0.005 |
| Grade III‐IV | 35 (24.5) | 6 (9.5) | 26 (33.8) | <0.005 | 13 (13.7) | 18 (43.9) | <0.005 |
| NASH—yes | 52 (33.8) | 26 (49.1) | 26 (40.0) | [0.324] | 38 (52.1) | 13 (31.7) | [0.036] |
| CASH—yes | 29 (18.8) | 20 (40.8) | 9 (15.5) | <0.005 | 25 (36.8) | 3 (8.3) | <0.005 |
| SOS—yes | 13 (8.4) | 10 (16.4) | 3 (4.3) | [0.021] | 13 (14.6) | 0 (0.0) | [0.009] |
| Steatosis (%) | 10.0 (0.0‐100.0) | 5.0 (0.0‐100.0) | 10.0 (0.0‐80.0) | [0.62] | 7.5 (0.0‐85) | 12.5 (0.0‐100.0) | [0.88] |
| Preoperative parameters | |||||||
| PT (%) | 101.0 (40.0‐150.0) | 103.0 (45.0‐150.0) | 97.0 (40.0‐150.0) | [0.071] | 103.0 (45.0‐150.0) | 93.0 (40.0‐150.0) | <0.005 |
| ALP (U/L) | 90.0 (38.0‐707.0) | 90.0 (38.0‐418.0) | 90.5 (42.0‐423.0) | [0.32] | 85.0 (38.0‐418.0) | 104.0 (48.0‐423.0) | <0.005 |
| GGT (U/L) | 68.0 (11.0‐1576.0) | 48.5 (11.0‐505.0) | 92.0 (13.0‐1335.0) | <0.005 | 50.0 (11.0‐710.0) | 157.0 (13.0‐1,335.0) | <0.005 |
| AST (U/L) | 31.0 (17.0‐224.0) | 29.0 (17.0‐71.0) | 34.0 (17.0‐224.0) | [0.024] | 28.0 (17.0‐113.0) | 52.0 (21.0‐224.0) | <0.005 |
| ALT (U/L) | 31.0 (7.0‐372.0) | 26.0 (7.0‐81.0) | 35.0 (8.0‐372.0) | [0.037] | 25.0 (7.0‐123.0) | 46.5 (8.0‐372.0) | <0.005 |
| Albumin (g/L) | 42.0 (30.2‐50.0) | 42.75 (34.0‐50.0) | 42.0 (30.2‐47.6) | [0.031] | 42.0 (34.0‐50.0) | 40.1 (30.2‐47.6) | [0.018] |
| Bilirubin (mg/dL) | 0.6 (0.1‐6.64) | 0.52 (0.0‐2.87) | 0.66 (0.0‐6.64) | <0.005 | 0.55 (0.0‐3.17) | 0.81 (0.0‐6.64) | [0.025 |
Abbreviations: 95% CI, 95% confidence interval; OR, odds ratio; SB, serum bilirubin.mCRC, metastatic colorectal cancer; HCC, hepatocellular cancer; CCC, cholangiocellular cancer; ICU, intensive care unit; NASH, non‐alcoholic steatohepatitis; CASH, chemotherapy associated steatohepatitis; SOS, sinusoidal obstruction syndrome; PT, prothrombin time; ALP, alkaline phosphatase; GGT, gamma‐glutamyl transpeptidase; AST, aspartate aminotransferase; ALT, alanine aminotransferasase.
The study was conducted in adherence to the Declaration of Helsinki and was approved by the Ethics Committee of the Medical University of Vienna. Ahead of participation, informed consent was obtained from all patients (EK Nr. 16‐253‐0117 and EK 14‐122‐0714).
Measurement of sAXL/GAS‐6, sMerTK, sCD163, IL‐6, CK18, and Routine Laboratory Parameters
Perioperative blood parameters of liver function were measured as part of the clinical routine by the local institutional laboratory and were documented prospectively (Table 1). Experimental parameters, including sAXL, sMerTK and GAS‐6, as well as sCD163, IL‐6 and CK18, were determined using enzyme‐linked immunosorbent assays. For a more detailed description of experimental procedures refer to the Supporting Methods section.
Statistical Analyses
IBM SPSS software version 20.0 (IBM Corp., Armonk, NY) and GraphPad Prism 8 (GraphPad Software, La Jolla, CA) were used for statistical data analyses, which was based on nonparametric testing for either paired or independent samples (Mann‐Whitney U test, Wilcoxon sign test, Spearman‐Rho, or Pearson correlation analysis). For the comparison of prevalence and incidence between the groups, chi‐squared tests were conducted.
The diagnostic validity of experimental parameters for LD was assessed by receiver operating characteristic (ROC) analysis considering the area under the curve (AUC). For preoperative risk stratification, cutoff values based on obtained data points were deduced using the Youden index. Calculation provided optimal cutoff values at concentrations, representing the highest sum of sensitivity and specificity and hence maximum discrimination in distinguishing high‐risk and low‐risk groups. P values < 0.05 were considered statistically significant.
Results
Patients With sAXLHIGH and GAS‐6HIGH Are at Risk of Postoperative LD and Worse Clinical Outcome
Initially, we evaluated the predictive potential of preoperative sAXL and GAS‐6 serum concentrations in patients undergoing liver resection. ROC analysis revealed distinct validity as preoperative markers for postoperative LD with an area under the curve (AUC) of 0.722 (sAXL, P < 0.005) and 0.721 (GAS‐6, P < 0.005) (Fig. 1A). Furthermore, their similarity in prediction as well as their functional association indicated significant correlation, which could be verified as illustrated in Supporting Fig. S2A. (R = 0.707, P < 0.005).
FIG. 1.
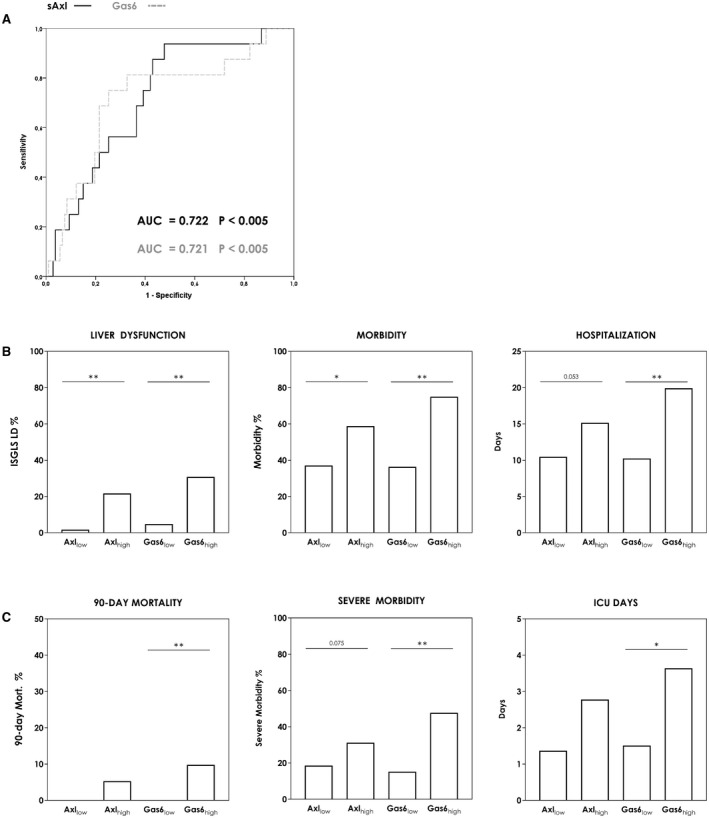
Prediction of postoperative outcome according to sAxl/Gas6 serum concentrations. Preoperative predictive value of sAxl and Gas6 for postoperative LD is demonstrated by ROC analysis (A). Classification according to deduced cutoffs (sAxl = 31.91 ng/mL; Gas6 = 34.42 mg/mL) demonstrates the variance of incidence among low‐risk and high‐risk subgroups for postoperative LD and morbidity (chi‐squared test) (B) as well as 90‐day mortality and severe postoperative morbidity (chi‐squared test) (C). Differences in postoperative and ICU stay are shown in (B) and (D) (two‐tailed unpaired Student t test). *P < 0.05; **P < 0.005. Abbreviation: ISGLS, International Study Group of Liver Surgery.
For improved risk stratification, we defined cutoff values according to respective ROC analysis (sAXL = 31.91 ng/mL; GAS‐6 = 34.42 mg/mL), enabling the most accurate identification of patients with high risk of postoperative LD (sAXL: sensitivity = 93.8%, specificity = 52.3%; GAS‐6: sensitivity = 75.0%, specificity = 74.8%). Patients with elevated GAS‐6 and sAXL levels were found to significantly differ in baseline characteristics (Table 1) and displayed a significantly higher frequency of cirrhosis and elevated liver function/damage parameters. Furthermore, in terms of postoperative outcome, the incidence of postoperative LD (sAXL: 1.67%‐21.74% [P < 0.005]; GAS‐6: 4.76%‐30.76% [P < 0.005]) and morbidity (sAXL: 37.14%‐58.75% [P = 0.008]; GAS‐6: 36.36%‐75.00% [P < 0.005]) was significantly increased in patients surpassing our cutoff values (Fig. 1B and Supporting Fig. S2B). In line with these findings, we observed a higher incidence of severe morbidity (sAXL: P = 0.075; GAS‐6: P < 0.005) and postoperative 90‐day mortality (sAXL: P = 0.123; GAS‐6: P = 0.007) in patients exceeding our preoperative sAXL and GAS‐6 cutoff values (Fig. 1C). These patients further displayed prolonged intensive care unit (ICU) (sAXL: P = 0.123; GAS‐6: P = 0.014) and hospital stay (sAXL: P = 0.053; GAS‐6: P < 0.005) (Fig. 1B,C).
When examining the prognostic value of sAXL and GAS‐6 levels in patients undergoing major liver resection, being of higher risk to develop postoperative complications, the trends were similar to the entire cohort. Obtained preoperative predictive validity was equal (sAXL AUC = 0.726 [P = 0.009] and GAS‐6 AUC = 0.726 [P = 0.009]) (Supporting Fig. S3A). Risk stratification, according to the prior chosen cutoffs, again demonstrated a significantly increased incidence of postoperative LD and morbidity within the high‐risk groups (LD: sAXL, 2.7%‐31.70% [P < 0.005]; GAS‐6, 7.69%‐41.67% [P < 0.005]; morbidity: sAXL, 43.48%‐63.27% [P = 0.053]; GAS‐6, 42.19%‐82.14% [P < 0.005]) (Supporting Fig. S3A). Preoperative sAXL and GAS‐6 concentrations were consequently consistent in the prediction of postoperative outcome regardless of further influencing/prognostic factors, except advanced fibrosis and cirrhosis, as sAXL performance appeared to be limited in these patients (low [fibrosis grade 0‐2]: sAXL, AUC = 0.763 [P = 0.005]; advanced [fibrosis grade 3‐4]: sAXL, AUC = 0.568 [P = 0.67]) (Supporting Fig. S3B). For GAS‐6, neither the degree of fibrosis (low [fibrosis grade 0‐2]: GAS‐6, AUC = 0.688 [P = 0.043]; advanced [fibrosis grade 3‐4]: GAS‐6, AUC = 0.795 [P = 0.065]) (Supporting Fig. S3B), nor resection extent strikingly altered the predictive value. For validation of their independence in the prediction of postoperative LD, a multivariate analysis was performed. Only sAXL and GAS‐6, as well as the extent of hepatic resection, remained significant predictors of postoperative LD. The results from the final model fit are given in Table 3. These data suggest that preoperative sAXL, except for advanced fibrosis, and GAS6 can predict postoperative outcomes regardless of the extent of resection and underlying grade of fibrosis. Unfortunately, the development of a model‐based prediction combining the benefits of both markers did not yield improved outcome prediction as visualized in Supporting Table S1. Limiting might be the strong correlation observed between sAXL and GAS6 (R = 0.707, P < 0.005) (Supporting Fig. S2A).
TABLE 3.
Multivariable Analysis for LD
| Parameter | Univariate Analysis | Multivariable Analysis sAxl | Multivariable Analysis Gas6 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| sAxl (ng/mL) | 1.049 | 1.013‐1.086 | 0.007 | 1.056 | 1.017‐1.096 | 0.005 | — | — | — |
| Gas6 (ng/mL) | 1.049 | 1.012‐1.088 | 0.010 | — | — | — | 1.062 | 1.020‐‐1.106 | 0.004 |
| Gender | 0.974 | 0.340‐2.794 | 0.961 | ||||||
| Neoplastic entity | 1.263 | 0.816‐1.955 | 0.294 | ||||||
| Hepatic resection | 6.154 | 1.352‐28.011 | 0.019 | 6.423 | 1.307‐31.576 | 0.022 | 7.580 | 1.428‐40.233 | 0.017 |
| Co‐factors | |||||||||
| Cirrhosis | 1.937 | 0.555‐6.761 | 0.300 | ||||||
| Fibrosis (≥ III) | 1.545 | 0.491‐4.868 | 0.457 | ||||||
| CASH | 0.429 | 0.089‐2.078 | 0.293 | ||||||
| SOS | 0.715 | 0.085‐6.056 | 0.759 | ||||||
| Steatosis (%) | 1.007 | 0.986‐1.028 | 0.545 | ||||||
| Preoperative parameters | |||||||||
| Platelets (×103/µL) | 0.997 | 0.990‐1.005 | 0.450 | ||||||
| Bilirubin (mg/dL) | 1.816 | 0.984‐3.351 | 0.056 | ||||||
| PT (%) | 0.994 | 0.969‐1.020 | 0.632 | ||||||
| ALP (U/L) | 1.006 | 1.001‐1.011 | 0.016 | 0.999 | 0.990‐1.008 | 0.824 | 0.998 | 0.990‐1.007 | 0.704 |
| GGT (U/L) | 1.002 | 1.000‐1.004 | 0.030 | 1.000 | 0.996‐1.004 | 0.972 | 1.000 | 0.996‐1.004 | 0.957 |
| AST (U/L) | 1.015 | 1.003‐1.027 | 0.013 | 0.999 | 0.982‐1.015 | 0.880 | 0.999 | 0.982‐1.017 | 0.940 |
| ALT (U/L) | 1.004 | 0.995‐1.012 | 0.417 | ||||||
| Albumin (g/L) | 0.864 | 0.758‐0.986 | 0.031 | 0.896 | 0.765‐1.050 | 0.175 | 0.921 | 0.786‐1.080 | 0.311 |
| Age (years) | 1.024 | 0.982‐1.067 | 0.268 | ||||||
Statistically significant values are shown in bold.
Abbreviations: 95% CI, 95% confidence interval; ALP, alkaline phosphatase; ALT, alanine aminotransferasase; AST, aspartate aminotransferase; CASH, chemotherapy associated steatohepatitis; GGT, gamma‐glutamyl transpeptidase; OR, odds ratio; PT, prothrombin time; SOS, sinusoidal obstruction syndrome.
sAXL and GAS‐6 Dynamics Following Partial Hepatectomy
Murine models indicate a central role of the receptor‐ligand pair AXL/GAS‐6 in liver regeneration. However, the perioperative dynamics in humans have never been evaluated. Given the significant association of sAXL/GAS‐6 with liver functional outcomes, we aimed to explore the dynamics of AXL/GAS‐6 levels in patients undergoing liver resection. In a subset of 63 patients (for baseline characteristics of the perioperative cohort refer Table 2), we therefore evaluated perioperative sAXL and GAS‐6 dynamics and could observe a steady rise of GAS‐6 serum level from preOP until POD5, while sAXL levels remained fairly stable (Fig. 2A,B).
TABLE 2.
Demographics of PeriOP Cohort
| Cohort Total (n = 63) | Cohort AXLlow (n = 23) | Cohort AXLhigh (n = 38) | Cohort GAS‐6low (n = 40) | Cohort GAS‐6high (n = 18) | |||
|---|---|---|---|---|---|---|---|
| n (%) – Median [Range] | |||||||
| Age (years) | 63.29 (24.33‐89.31) | 60.47 (31.97‐89.21) | 65.06 (37.18‐86.14) | [0.326] | 61.27 (31.97‐89.21) | 67.72 (46.9‐81.21) | [0.305] |
| Sex | |||||||
| Male | 41 (65.1) | 14 (60.9) | 26 (68.4) | [0.547] | 26 (65.0) | 11 (61.1) | [0.776] |
| Female | 22 (34.9) | 9 (39.1) | 12 (31.6) | [0.547] | 14 (35.0) | 7 (38.9) | [0.776] |
| Neoplastic entity | |||||||
| mCRC | 10 (15.9) | 8 (34.8) | 2 (5.3) | <0.005 | 8 (20.0) | 2 (11.1) | [0.708] |
| HCC | 22 (34.9) | 2 (8.7) | 19 (50.0) | <0.005 | 11 (27.5) | 9 (50.0) | [0.095] |
| CCC | 22 (34.9) | 7 (30.4) | 14 (36.8) | [0.61] | 12 (30.0) | 7 (38.9) | [0.505] |
| Non‐neoplastic | 7 (11.1) | 5 (21.7) | 2 (5.3) | [0.093] | 7 (17.5) | 0 (0.0) | [0.087] |
| Other | 2 (2.3) | 1 (4.3) | 1 (2.6) | [1.0] | 2 (5.0) | 0 (0.0) | [1.0] |
| Resection extent | |||||||
| Major | 52 (82.5) | 21 (91.3) | 29 (76.3) | [0.182] | 34 (85.0) | 15 (83.3) | [1.0] |
| Minor | 11 (17.5) | 2 (8.7) | 9 (23.7) | [0.182] | 6 (15.0) | 3 (16.7) | [1.0] |
| Outcome | |||||||
| Postoperative LD—yes | 11 (20.0) | 0 (0.0) | 10 (30.3) | [0.008] | 2 (5.9) | 7 (43.8) | <0.005 |
| Morbidity—yes | 34 (54.0) | 10 (43.5) | 22(57.9) | [0.275] | 18 (45.0) | 13 (72.2) | [0.055] |
| Severe morbidity—yes | 18 (28.6) | 4 (17.4) | 13 (34.2) | [0.156] | 7 (17.5) | 9 (50.0) | [0.024] |
| 90‐day mortality—yes | 1 (1.8) | 0 (0.0) | 1 (2.9) | [1.0] | 0 (0.0) | 1 (5.9) | [0.321] |
| ICU days | 1.0 (0.0‐26.0) | 1.0 (0.0‐5.0) | 1.0 (0.0‐26.0) | [0.045] | 1.0 (0.0‐10.0) | 2.0 (0.0‐26.0) | [0.029] |
| Hospitalization days | 10.0 (3.0‐117.0) | 8.0 (4.0‐46.0) | 11.5 (3.0‐117.0) | [0.512] | 8.0 (3.0‐46.0) | 14.0 (5.0‐ 117.0) | [0.016] |
| Liver histology | |||||||
| Fibrosis—yes | 42 (75.0) | 13 (68.4) | 28 (77.8) | [0.522] | 26 (70.3) | 12 (80.0) | [0.731] |
| Grade 0‐II | 41 (73.2) | 16 (84.2) | 25 (69.4) | [0.334] | 31 (83.3) | 10 (66.7) | [0.26] |
| Grade III‐IV | 15 (26.8) | 3 (15.8) | 11 (30.6) | [0.334] | 6 (16.2) | 5 (33.3) | [0.26] |
| NASH—yes | 19 (43.2) | 9 (64.3) | 10 (35.7) | [0.079] | 12 (52.2) | 5 (31.2) | [0.195] |
| CASH—yes | 7 (18.9) | 6 (46.2) | 1 (4.5) | [0.006] | 6 (28.6) | 0 (0.0) | [0.071] |
| SOS—yes | 3 (5.7) | 1 (5.0) | 2 (6.5) | [1.0] | 3 (8.6) | 0 (0.0) | [0.378] |
| Steatosis (%) | 5.0 (0.0‐100.0) | 5.0 (0.0‐100.0) | 10.0 (0.0‐80.0) | [0.634] | 5.0 (0.0‐85.0) | 17.5 (0.0‐80.9) | [0.361] |
| Preoperative parameters | |||||||
| PT (%) | 101.0 (62.0‐150.0) | 106.0 (83.0‐136.0) | 99.0 (62.0‐150.0) | [0.275] | 104.0 (80.0‐137.0) | 94.0 (62.0‐150.0) | [0.05] |
| ALP (U/L) | 89.0 (38.0‐423.0) | 98.0 (38.0‐169.0) | 88.0 (42.0‐423.0) | [0.731] | 86.5 (38.0‐230.0) | 90.5 (51.0‐423.0) | [0.353] |
| GGT (U/L) | 67.0 (11.0‐710.0) | 54.0 (11.0‐505.0) | 82.0 (13.0‐710.0) | [0.354] | 54.0 (11.0‐710.0) | 142.0 (13.0‐562.0) | [0.044] |
| AST (U/L) | 28.0 (17.0‐208.0) | 27.0 (19.0‐51.0) | 30.0 (17.0‐208.0) | [0.43] | 27.0 (17.0‐113.0) | 45.0 (22.0‐208.0) | <0.005 |
| ALT (U/L) | 27.0 (9.0‐372.0) | 26.0 (11.0‐78.0) | 27.0 (9.0‐372.0) | [0.523] | 25.0 (10.0‐123.0) | 42.0 (9.0‐372.0) | [0.017] |
| Albumin (g/L) | 42.0 (30.2‐48.0) | 43.5 (38.0‐48.0) | 42.0 (30.2‐47.6) | [0.06] | 42.0 (36.0‐48.0) | 40.0 (30.2‐47.6) | [0.251] |
| Bilirubin (mg/dL) | 0.63 (0.29‐6.64) | 0.49 (0.29‐1.09) | 0.9 (0.34‐6.64) | <0.005 | 0.55 (0.29‐3.17) | 1.0 (0.42‐6.64) | [0.041] |
Abbreviation: 95% CI, 95% confidence interval; ALP, alkaline phosphatase; ALT, alanine aminotransferasase; AST, aspartate aminotransferase; CASH, chemotherapy associated steatohepatitis; CCC, cholangiocellular cancer; GGT, gamma‐glutamyl transpeptidase; HCC, hepatocellular cancer; ICU, intensive care unit; mCRC, metastatic colorectal cancer; NASH, non‐alcoholic steatohepatitis; OR, odds ratio; PT, prothrombin time; SOS, sinusoidal obstruction syndrome.
FIG. 2.
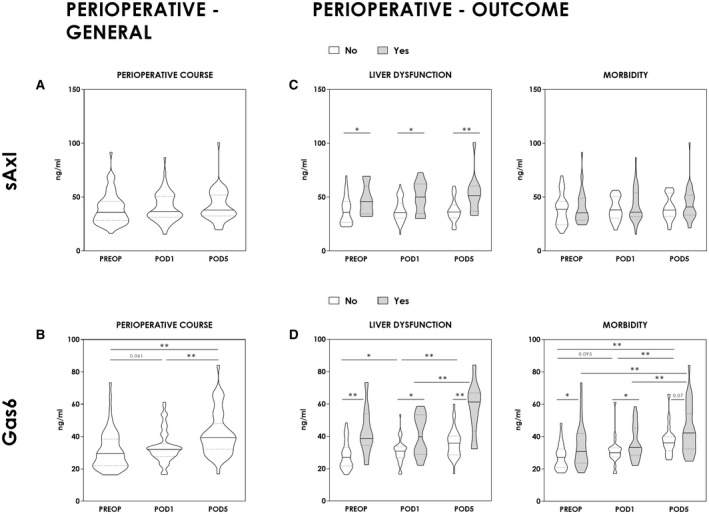
Perioperative time course. sAxl and Gas6 concentrations were measured preoperatively (PREOP), on postoperative day 1 (POD1), and postoperative day 5 (POD5). Perioperative dynamic is illustrated in general (Mann‐Whitney U test and Wilcoxon signed‐rank test) (A,B) as well as separately for LD and postoperative morbidity (Mann‐Whitney U test and Wilcoxon signed‐rank test) (C,D). *P < 0.05; **P < 0.005.
Major resections, which require more intensive liver regeneration, showed an even more pronounced dynamic, whereas patients undergoing minor resection were missing a significant increase following liver resection (Supporting Fig. S4).
Most importantly, we could observe outcome‐dependent postoperative discrepancies, in particular for GAS‐6, whereas sAXL concentrations showed only limited postoperative outcome‐related dynamics. In general, measured concentrations were distinctly higher in patients with LD for GAS‐6 (PREOP, P < 0.005; POD1, P = 0.041; POD5, P < 0.005) and sAXL (PREOP, P = 0.033; POD1, P = 0.016; POD5, P = 0.004) (Fig. 2C,D). Furthermore, serum concentrations of GAS‐6 overall rose until POD5, but far more pronounced in patients with postoperative LD (P = 0.005) (Fig. 2D). However, the immediate GAS‐6 response in patients with LD appeared to be blunted, as we observed a less pronounced initial release on POD1 than in patients without postoperative LD (increase on POD1 only in patients with no LD [P = 0.029]) (Fig. 2D).
As a clinical hallmark of postoperative LD, the evaluation of postoperative morbidity completed our analysis. Trends in postoperative morbidity paralleled the association of GAS‐6 and the incidence of liver dysfunction. Postoperative morbidity was significantly associated with higher GAS‐6 concentrations (PREOP, P = 0.02; POD1, P = 0.033; POD5, P = 0.07) (Fig. 2D). Additionally, the perioperative course showed an initial slight increase until POD1, except for patients with postoperative morbidity (PREOP‐POD1: Morb., P = 0.329; No Morb., P = 0.095). This was followed by a steep increase in GAS‐6 concentrations peaking at POD5 and, similar to patients with LD, more pronounced in the case of postoperative morbidity (POD1‐POD5: Morb., P < 0.005; No Morb., P < 0.005) (Fig. 2D). Thus, sAXL/GAS‐6 are not only predictive of postoperative outcomes following liver resection, but also observed postoperative dynamics suggest a distinct pattern of response associated with human liver regeneration.
Immediate Rise of sAXL and GAS‐6 as Indication of their Contribution to Liver Regeneration
Distinct postoperative dynamics of sAXL and GAS‐6 with regard to the extent of liver resection as well as the fact that patients with LD and morbidity failed to increase on POD1 led us to hypothesize that patients with underlying liver disease might suffer from prior chronic and consequently exhaustive activation of AXL/GAS‐6 signaling, especially because patients with preoperatively elevated concentrations, as found in the context of CLD, were unable to respond with a further boost in the signaling activity after induction of liver regeneration.
Consequently, we aimed to investigate circulating sAXL and GAS‐6 dynamics in more detail, namely immediately after induction of liver regeneration. To address alterations in this early period, we analyzed liver vein samples taken 2 hours after induction of liver regeneration. Indeed, we could observe a significant increase of sAXL and GAS‐6 levels immediately following induction of liver regeneration (i.e., 2 hours after portal vein ligation) (Fig. 3). When patients were again classified based on the previously defined cutoff values, we observed consistently higher sAXL and GAS‐6 levels in patients with preexisting elevation of sAXL and GAS‐6 (sAXL, P = 0.014 [Fig. 3A]; GAS‐6, P = 0.012 [Fig. 3B]). However, again subgroups with high preoperative sAXL and GAS‐6 concentrations did lack an immediate induction during this very early time point in human liver regeneration (Preop–liver vein: sAXL, P = 0.263 [Fig. 3A]; GAS‐6, P = 0.028 [Fig. 3B]). Overall, serum concentrations even tended to decline in patients with high preoperative sAXL and GAS‐6, whereas patients with no elevation preoperatively remained fairly stable or increased (fold change: sAXLhigh [preop] vs. sAXLlow [preop], P = 0.017 [Fig. 3A]; GAS‐6high [preop] vs. GAS‐6low [preop], P = 0.012 [Fig. 3B]).
FIG. 3.
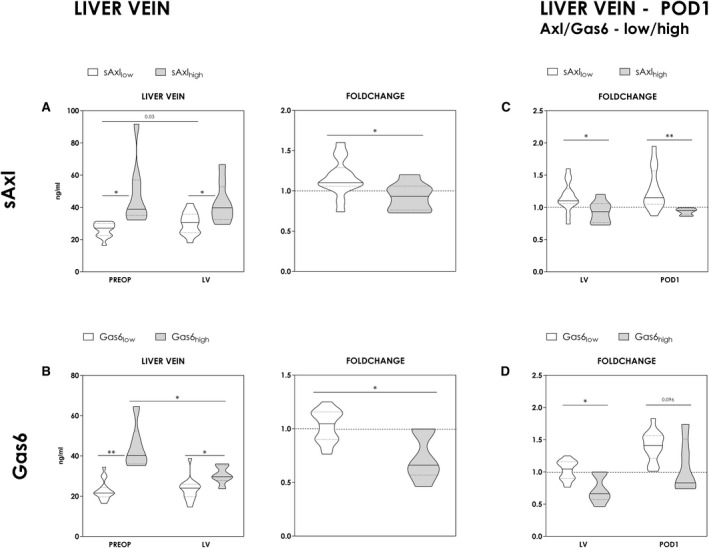
Dynamics of sAxl and Gas6 during early liver regeneration. To evaluate alterations in the early phase, sAxl and Gas6 levels were assessed in the liver vein (LV) of the regenerating liver lobe 2 hours after induction of regeneration and on POD1. Results are shown in relation to the preoperative concentrations to exemplify postresection alterations. Postoperative changes are further indicated by corresponding fold change (concentrations in the LV divided by baseline concentrations [PREOP]). (A,B) Observed dynamics [absolute and fold change] in the LV and on POD1 were compared between high‐risk and low‐risk groups (Mann‐Whitney U test and Wilcoxon signed‐rank test) (A,B & C,D). Classification was based on antecedently chosen cutoff values. (Mann‐Whitney U test). *P < 0.05; **P < 0.005.
Observed tendencies continued up to POD1, as illustrated in Fig. 3C,D. Serum concentrations of sAXL or GAS‐6 only rose in patients without preoperatively elevated sAXL/GAS‐6 levels (fold change: sAXLhigh [preop] vs. sAXLlow [preop], P < 0.005; GAS‐6high [preop] vs. GAS‐6low [preop], P = 0.096). These data further support the hypothesis that an acute dynamic response in AXL/GAS‐6 signaling could be required for functional liver regeneration.
Diminished M2 Polarization in Patients With GAS‐6HIGH
As we had further substantiated the observation that patients with exaggerated preoperative AXL/GAS‐6‐signaling appeared to show chronic exhaustion of this immunoregulatory pathway, we also aimed to assess intrahepatic inflammatory changes in more detail. Resident macrophages (Kupffer cells) might be of relevance, as GAS‐6 mediated AXL signaling/clearance of apoptotic cells has been shown to favor a regenerative macrophage phenotype that is essential for the resolution of inflammation.
These so‐called M2 macrophages express high levels of CD163, which is cleaved and shed into the circulation. Accordingly, CD163 has been proposed as a surrogate parameter for an M2 phenotypical switch in macrophages.( 28 , 29 ) In line with these findings, we observed a significant association of high sAXL and GAS‐6 concentrations and elevated sCD163, suggesting a pronounced M2 polarization (Fig. 4) Interestingly, however, patients with preoperatively high concentrations failed to increase circulating sCD163 immediately after induction of liver regeneration, suggesting a reduced shift in the M2 macrophage phenotype. Furthermore, a sparse increase postoperatively, as observed in patients with high preoperative concentrations, was associated with decreased soluble CD163 concentrations (fold change of AXL and fold change of sCD163: R = 0.616, P = 0.033 [Fig. 4B]); fold change of GAS‐6 and fold change of sCD163: R = 0.607], P = 0.036 [Fig. 4A]) (Fig. 4). In addition, MerTK, another receptor of the TAM‐RTK family, is prominently expressed on macrophages, including Kupffer cells. Similar to AXL, it is considered relevant for M2 polarization and sufficient regeneration after acute liver damage.( 24 ) Interestingly, no perioperative dynamics could be observed and overall concentrations were low, whereas for sAXL an increase could be noted (Fig. 4B). Accordingly, no significant association with sCD163 could be observed, neither with absolute concentrations nor with postoperative alterations (Fig. 4B). Overall, data obtained for GAS6 were most significant, possibly due to its solubility as well as affinity to AXL and MerTK.
FIG. 4.
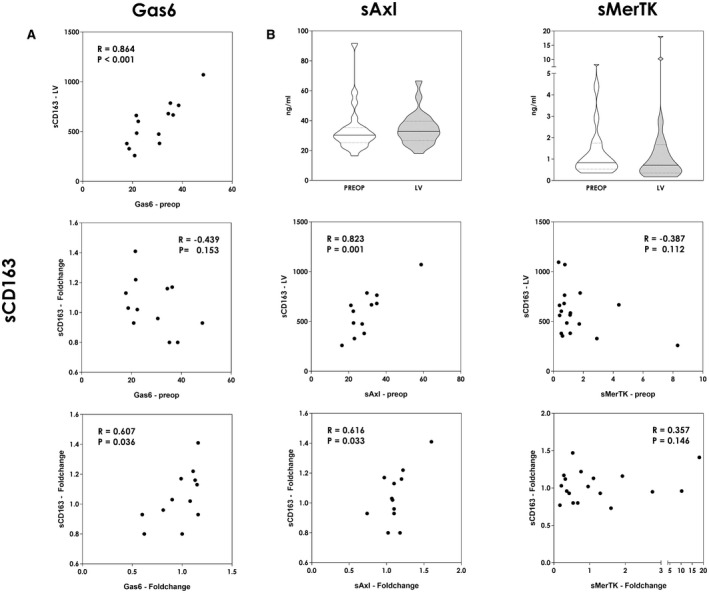
Postoperative M2 polarization. Correlation of Gas6, sAxl, and MerTK concentrations with sCD163. Analyses were performed for absolute values (in nanograms per milliliter [ng/mL]) as well as the postoperative fold change (LV divided by baseline concentrations [PREOP]) (A,B). sAxl as well as sMerTK concentrations are shown for the immediate perioperative time course [PREOP and 2h after resection in the LV] (Mann‐Whitney U test) (B). *P < 0.05; **P < 0.005.
As a limited M2 phenotypical switch of macrophages has been associated with pronounced inflammation, we further assessed pro‐inflammatory cytokine concentrations immediately after induction of liver regeneration.
Elevated IL‐6 and CK18 as Indicators of Pronounced and Overwhelming Inflammation
A phenotypical switch in favor of M2 polarization is also accompanied by a change in cytokine profile. Anti‐inflammatory cytokines are up‐regulated at the expense of pro‐inflammatory cytokines such as IL‐6. Accordingly, we observed a pronounced release of IL‐6 following liver resection and consequently also significantly higher circulating levels on POD1 in patients with preoperatively elevated GAS‐6 concentrations (Fig. 5A). Because extensive inflammation results in cellular demise, we further evaluated CK18 as a surrogate marker for intrahepatic cell death. To assess the proportional amount of necrosis and apoptosis, both the caspase‐cleaved CK18 (cCK18) by detection of the M30 epitope (CK18/30) and the full‐length protein CK18 were determined. Because a selective measurement of the uncleaved full‐length form is not possible, M5 and M6 epitopes (K18/65) are present on the caspase‐cleaved protein as well; the difference of CK18/65 and CK18/30 indicates the amount of necrosis. Both elevated CK18/30 and CK18/65 were associated with high GAS‐6 concentrations preoperatively as well as on POD1, and both rose significantly up to POD1 (Fig. 5B,C). However, contrary to CK18/30, we observed a significant difference in the postoperative increase of CK18/65, comparing patients with low and high preoperative GAS‐6 concentrations. High GAS‐6 concentrations before surgery and, consequently, a diminished GAS‐6 burst were associated with a significantly more pronounced release of CK18/65 (Fig. 5C). Hence, observed differences indicated elevated cell death in the form of necrosis under pronounced inflammatory conditions, as found in patients with insufficient GAS‐6 release following LR.
FIG. 5.
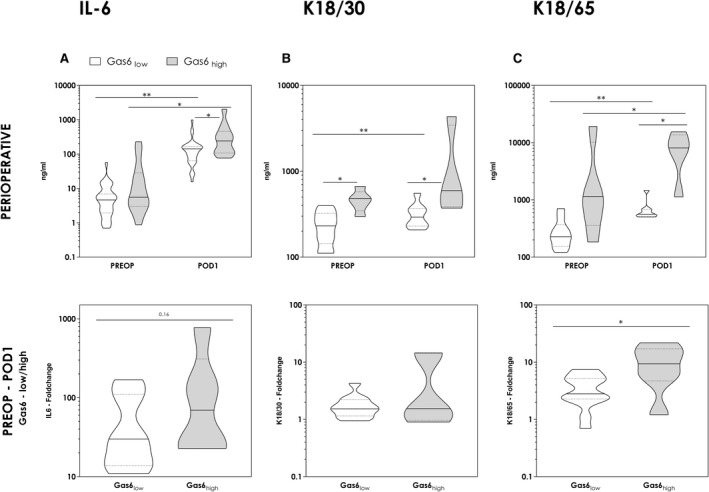
Pronounced postoperative inflammation and cellular demise in Gas6high patients. To evaluate postoperative inflammation and resultant cell death, IL‐6 concentrations and cytokeratins were measured in the perioperative course. Results are displayed comparing Gas6low and Gas6high patients. (Mann‐Whitney U test and Wilcoxon signed‐rank test). For an exact evaluation of postoperative dynamics, observed alterations were evaluated and illustrated as fold change of IL‐6 as well as CK18/30 and CK18/65 (fold change = concentrations on POD1 divided by baseline concentrations [PREOP]) (Mann‐Whitney U test). *P < 0.05; **P < 0.005.
Discussion
Within this study, we aimed to assess the relevance of the AXL/GAS‐6 pathway in liver surgery and regeneration. Initially, we found that patients with preoperatively increased sAXL and GAS‐6 levels suffered from postoperative LD and adverse clinical outcome significantly more frequently. Given the sensitive quantification of underlying liver disease, hepatic function and liver damage, these data provide strong exploratory evidence for their clinical suitability as biomarkers. However, even though patients suffering from LD were found to have elevated sAXL and GAS‐6 levels during the perioperative period, presumably caused by chronic activation via ongoing intrahepatic inflammation, these patients appeared to suffer from the exhaustion of this mechanism, as they failed to respond with an adequate “burst” immediately after the induction of liver regeneration. This rapid rise following liver resection, particularly regarding GAS‐6, appeared to be essential, as it was not only associated with intrahepatic immunomodulatory macrophage activation but also resulted in a reduction of pro‐inflammatory signaling during the early phase of liver regeneration. Therefore, our data provide distinct evidence for the critical relevance of this pathway in balancing inflammatory processes and counteracting overshooting intrahepatic inflammation during the priming phase of liver regeneration, as summarized in the graphical abstract.
Because adequate clinical management of insufficient regeneration and resulting LD is still limited, preoperative risk stratification is of critical importance to avoid this often fatal complication. Specifically in patients with underlying liver disease, markers to predict the ability of the remnant liver to regenerate after liver resection remain very limited. Only a few tests, invasive as well as noninvasive, were considered suitable for clinical application and hence have been clinically implemented. Limiting are especially cost efficiency, general availability, and invasiveness. Accordingly, circulating biomarkers appear to be an elegant solution.( 30 ) Considering low costs and easy applicability, an implementation is feasible and enables broad accessibility. In this context, sAXL and GAS‐6 are promising candidates. Recent studies demonstrated excellent predictive value regarding CLD.( 15 , 16 , 31 ) However, we report their promising predictive potential for postoperative LD in patients undergoing liver surgery. Accordingly, we found that elevated preoperative sAXL and GAS‐6 levels were directly associated with significantly worse clinical outcome after liver resection. Furthermore, both parameters are strong in prediction and (in terms of GAS‐6) independent from underlying liver disease (Table 3 [multivariate analysis] and Supporting Fig. S3). Their assessment hence represents a valuable tool for preoperative risk evaluation. However, while we observed rather close associations of sAXL and GAS‐6 with clinical outcome in a considerable amount of patients, prospective validation of these results is of utmost importance.
Kupffer cells release multiple cytokines and mediators, including TNF‐α, IL‐6 and IL‐1β, which have been shown to represent important inducers of liver regeneration.( 7 , 32 ) Although these factors are certainly of importance as initial stimulus of liver regeneration, experimental models have also documented the necessity of tight regulation of these processes. For example, the simple duration of IL‐6 exposure was found to critically affect liver repair in mice, with adverse effects of prolonged exposure,( 33 ) but Kupffer cells do not simply exert pro‐inflammatory functions. The concept of M1 and M2 polarization has changed the understanding of tissue‐resident macrophages and Kupffer cells in particular. Multiple studies have now documented a very dynamic phenotypical plasticity. In addition to their pro‐inflammatory M1 phenotype, they further exhibit the capability to express anti‐inflammatory, pro‐regenerative cytokines, including IL‐10, IL‐13 or transforming growth factor beta (TGF‐β),( 7 , 32 , 34 , 35 ) reflecting a phenotypical switch to an M2‐like state.( 21 , 36 ) Tissue‐resident, as well as migrated macrophages, may therefore evidently account for the initial pro‐inflammatory stimulus of liver regeneration, while also tightly regulating intrahepatic inflammation. In this context, sCD163 represents a circulating monocyte/macrophage‐specific marker, which we found increased immediately after the induction of liver regeneration in our patients. As M2 macrophages express high levels of CD163,( 28 ) and the fact that CD163‐expressing macrophages are frequently found in areas of regenerating tissue after injury,( 37 ) its soluble form has been proposed as a marker, indicating macrophage phenotypical switch toward M2 polarization.( 38 , 39 ) This is in line with a growing body of evidence suggesting that CD163 represents an anti‐inflammatory molecule.( 40 )
The regulation of this phenotypical switch is certainly multifactorial. However, TAM signaling (such as the AXL/GAS‐6 pathway) has been shown to critically affect this process as well as liver regeneration itself. Impaired TAM‐receptor surface cleavage in mice with bleomycin‐induced lung injury reduced inflammation and apoptosis, due to a shift in the cytokine profile. In particular, predominate expression of pro‐regenerative and anti‐inflammatory factors such as TGF‐β, as compared with pro‐inflammatory cytokines such as TNF‐α and IL‐1β, resembles a picture of a phenotypical macrophage switch to an M2‐like state.( 41 ) Similarly, experimental data associated absent TAM‐signaling after acute liver injury with a profound and overwhelming inflammatory response.( 23 , 24 ) In this context, macrophage‐mediated immunomodulation might be of particular relevance. The anti‐inflammatory effect of GAS‐6 observed in a murine model for hepatic reperfusion/ischemia could be reproduced in a surrogate Kupffer cell line. Administration of GAS‐6 attenuated pro‐inflammatory stimuli, as TNF‐α and IL‐1β were distinctly down‐regulated by serine/threonine‐protein kinase–mediated prevention of nuclear factor kappa B (NF‐κβ) activation, shifting Kupffer cells from an M1 to an M2‐like phenotype.( 23 ) Similarly, other studies report on the GAS‐6‐mediated regulation of NF‐κB in macrophages.( 42 ) Furthermore, Rothlin et al. proposed AXL signaling as part of a counterregulatory feedback loop in response to pro‐inflammatory stimuli, as the initial signal transducer and activator of transcription (STAT)–mediated inflammatory response provokes an up‐regulation of AXL and hence TAM‐signaling.( 18 , 43 ) Favoring an anti‐inflammatory macrophage phenotype, TAM signaling reduces pro‐inflammatory M1‐like macrophages as well as pro‐inflammatory cytokine expression.( 21 , 36 , 41 ) M2 macrophages further exert anti‐inflammatory and pro‐regenerative influence by the release of, for example, TGF‐β, IL‐4, and IL‐10.( 7 , 32 , 34 , 35 ) These might, in turn, drive GAS‐6 release as reported by Nepal et al. and consequently sustain a balancing circuit in auto and paracrine manner.( 18 , 44 ) In line with these results, GAS‐6‐knockout animals showed insufficient regeneration and more frequently succumbed to fulminant hepatic failure in a model of acute liver injury.( 23 ) Similarly, we observed a distinct dysregulation of the immediate induction during early liver regeneration in patients with preoperatively elevated GAS‐6 levels. In particular, an adequate uprise (“burst”) after induction of liver regeneration appeared to be of critical relevance for efficient liver regeneration in our patients. Despite sparse knowledge about TAM‐receptor shedding and its implications, it may be assumed that cellular up‐regulation translates into increased serum concentrations. Importantly, shedding occurs following activation.( 24 ) In accordance, Zagorska et al. reported prominent AXL activation following acute Jo2‐induced (Fas‐agonistic antibody‐mediated) liver injury in mice. Consequent cleavage was indicated by the appearance of its soluble domain 2 hours after Jo2 injection. Interestingly, MerTK was less affected, which might explain our results of overall low concentrations and the less pronounced postoperative dynamic (Fig. 4B). However, no exact conclusion regarding MerTK activation could be drawn from these experimental analyses, as MerTK knockout was detrimental in the context of liver injury, even though less pronounced than AXL knockout.( 24 )
In the context of TAM‐receptor signaling, M2 polarization might be of particular relevance. MerTK, which is strongly expressed on macrophages, including Kupffer cells, was previously linked to M2 polarization.( 24 , 45 ) However, Kupffer cells, other than most resident macrophages, have also been shown to express high levels of AXL.( 24 ) As we did not observe any association of MerTK with CD163, whereas sAXL and GAS6 were found to correlate with postoperative CD163 dynamics (Fig. 4), the AXL/GAS6 axis might have increased relevance in M2‐macrophage switch as well as hepatic regeneration.( 24 ) However, the exact mechanism will have to be explored in consecutive experimental analyses. Furthermore, it has to be considered that also hepatic stellate cells, which are activated by TAM‐receptor signaling, especially via AXL/GAS6, might significantly contribute to hepatic regeneration. Following acute liver injury, hepatic stellate cell (HSC) activation is considered a physiological response mechanism promoting tissue repair. Concomitant GAS6 up‐regulation observed in vitro,( 46 ) as well as established TAM‐receptor up‐regulation in HSC,( 24 , 46 ) might, in addition to Kupffer cells, account for the sAXL and GAS6 increase observed in patients undergoing liver resection as well as represent an additional critical mechanism of action of this pathway.
Intriguingly, patients with preoperatively elevated sAXL and GAS‐6 concentrations, appear to lack sufficient up‐regulation, suggesting exhaustion of this acute response in the context of CLD. Reduced liver regeneration in patients with underlying liver disease is clinically well established. However, mechanistic principles behind are only poorly understood, and existing rodent models of liver regeneration almost exclusively focus on healthy livers. Indeed, in the context of underlying liver disease, chronic inflammatory processes and, in particular, TAM signaling (such as the AXL/GAS‐6 pathway), might be of distinct relevance.( 47 ) Chronic exhaustive activation of this counterregulatory mechanism has been described in patients with CLD and might restain an additional increase upon acute liver injury and consequently regeneration.( 17 ) A loss of particular mechanism, however, is detrimental, as in several murine models with TAM receptor as well as ligand knockout following acute injury increased postoperative inflammation and cellular demise could be noted.( 23 , 24 )
Consequently, we could demonstrate a significant association of sAXL as well as GAS‐6 expression and proinflammatory cytokine IL‐6. Along with an insufficient rise of sAXL and GAS‐6, increased IL‐6 expression could be noted (Fig. 5). These patients appeared to suffer from excessive immune activation following liver resection. The extent of TAM signaling may be inadequate to curb pro‐inflammatory processes to an optimal level, granting regeneration but avoiding intrahepatic cell damage. As we could demonstrate, this vacant resolution of postoperative inflammation, in the end, results in excessive cell demise. Significantly elevated levels of CK18 (intact full length) indicated pronounced cell death. CK18 is an intermediate filament important for epithelial and liver cells’ structural integrity, which is released upon cellular demise, and multiple previous reports indicated predictive value in terms of acute liver injury.( 48 , 49 ) While its caspase‐cleaved form (cCK18) accumulates during apoptosis, the release of the full‐length protein is necrosis‐associated.( 50 ) cCK18 is measured selectively by detection of the M30 epitope, which is exposed by proteolytic cleavage during apoptosis. The two other common epitopes for detection are M6 and M5 (CK18/65). Present on both the full‐length protein and caspase‐cleaved fragment, these are postulated as a general indicator of cellular demise.( 49 , 50 ) Interestingly, we could not observe a significant difference regarding the amount of apoptosis (CK18/30) comparing GAS‐6low and GAS‐6high cohorts, whereas CK18/65 concentrations rose significantly more in patients with high preoperative GAS‐6 levels. Consequently, these patients suffered from more intrahepatic cellular demise in general, considering the equivalent rise of apoptosis from pronounced nonapoptotic cell death, presumably necrosis.
In conclusion, this study provides distinct evidence for the relevance of AXL and GAS‐6 in hepatic immunomodulation, and thus for human liver regeneration. Preoperatively elevated levels were associated with preexisting liver disease, presumably caused by chronic intrahepatic inflammation. Consequently, we demonstrate the suitability of sAXL and GAS‐6 as predictive markers for the postoperative outcome, based on a large cohort of patients. Considering the advantages of blood‐prone biomarkers in terms of cost efficiency and accessibility, sAXL and GAS‐6 might represent a valuable tool for preoperative evaluation of liver function and regenerative reserve. If validated, a clinical implementation may help in the identification of patients at high risk, and thus provide a possibility to individualize treatment strategies.
In addition, we could demonstrate an immediate increase of sAXL and GAS‐6 following liver resection. However, patients who displayed an inadequate rise in sAXL or GAS‐6 were found to respond with overshooting intrahepatic inflammation. The gathered evidence suggests that the experimentally documented M1/M2 phenotypical switch of Kupffer cells is critical in this process. Given promising experimental treatment options for the manipulation of TAM‐receptor signaling, these translational data provide a central foundation for the development of therapeutic strategies also in humans. Because no therapeutic options are currently available for patients who develop postoperative LD, this might be of critical relevance.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Supplementary Material
Supported by the National Institutes of Health (R01DK122813), the Medical Scientific Fund of the Mayor of the City of Vienna (P‐19098), and the Austrian Science Fund (P‐32064).
Potential conflict of interest: Nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1. Clavien P‐A, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med 2007;356:1545‐1559. [DOI] [PubMed] [Google Scholar]
- 2. Dahm F, Georgiev P, Clavien P‐A. Small‐for‐size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant 2005;5:2605‐2610. [DOI] [PubMed] [Google Scholar]
- 3. Forbes SJ, Newsome PN. Liver regeneration—mechanisms and models to clinical application. Nat Rev Gastroenterol Hepatol 2016;13:473‐485. [DOI] [PubMed] [Google Scholar]
- 4. Capussotti L, Viganò L, Giuliante F, Ferrero A, Giovannini I, Nuzzo G. Liver dysfunction and sepsis determine operative mortality after liver resection. Br J Surg 2009;96:88‐94. [DOI] [PubMed] [Google Scholar]
- 5. Holt MP, Cheng L, Ju C. Identification and characterization of infiltrating macrophages in acetaminophen‐induced liver injury. J Leukoc Biol 2008;84:1410‐1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wen Y, Lambrecht J, Ju C, Tacke F. Hepatic macrophages in liver homeostasis and diseases—diversity, plasticity and therapeutic opportunities. Cell Mol Immunol 2021;18:45‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campion SN, Johnson R, Aleksunes LM, Goedken MJ, van Rooijen N, Scheffer GL, et al. Hepatic Mrp4 induction following acetaminophen exposure is dependent on Kupffer cell function. Am J Physiol Gastrointest Liver Physiol 2008;295:G294‐G304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meijer C, Wiezer MJ, Diehl AM, Yang S‐Q, Schouten HJ, Meijer S, et al. Kupffer cell depletion by CI2MDP‐liposomes alters hepatic cytokine expression and delays liver regeneration after partial hepatectomy. Liver 2000;20:66‐77. [DOI] [PubMed] [Google Scholar]
- 9. Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int 2006;26:1175‐1186. [DOI] [PubMed] [Google Scholar]
- 10. Dixon LJ, Barnes M, Tang H, Pritchard MT, Nagy LE. Kupffer cells in the liver. Compr Physiol 2013;3:785‐797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Das A, Sinha M, Datta S, Abas M, Chaffee S, Sen CK, et al. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol 2015;185:2596‐2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wan J, Benkdane M, Teixeira‐Clerc F, Bonnafous S, Louvet A, Lafdil F, et al. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology 2014;59:130‐142. [DOI] [PubMed] [Google Scholar]
- 13. Li M, Sun X, Zhao J, Xia L, Li J, Xu M, et al. CCL5 deficiency promotes liver repair by improving inflammation resolution and liver regeneration through M2 macrophage polarization. Cell Mol Immunol 2020;17:753‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Markose D, Kirkland P, Ramachandran P, Henderson NC. Immune cell regulation of liver regeneration and repair. J Immunol Regen Med 2018;2:1‐10. [Google Scholar]
- 15. Staufer K, Dengler M, Huber H, Marculescu R, Stauber R, Lackner C, et al. The non‐invasive serum biomarker soluble Axl accurately detects advanced liver fibrosis and cirrhosis. Cell Death Dis 2017;8:e3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bárcena C, Stefanovic M, Tutusaus A, Joannas L, Menéndez A, García‐Ruiz C, et al. Gas6/Axl pathway is activated in chronic liver disease and its targeting reduces fibrosis via hepatic stellate cell inactivation. J Hepatol 2015;63:670‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mukherjee SK, Wilhelm A, Antoniades CG. TAM receptor tyrosine kinase function and the immunopathology of liver disease. Am J Physiol Gastrointest Liver Physiol 2016;310:G899‐G905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 2007;131:1124‐1136. [DOI] [PubMed] [Google Scholar]
- 19. Lemke G. Biology of the TAM receptors. Cold Spring Harb Perspect Biol 2013;5:a009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anderson HA, Maylock CA, Williams JA, Paweletz CP, Shu H, Shacter E. Serum‐derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells. Nat Immunol 2003;4:87‐91. [DOI] [PubMed] [Google Scholar]
- 21. Myers KV, Amend SR, Pienta KJ. Targeting Tyro3, Axl and MerTK (TAM receptors): implications for macrophages in the tumor microenvironment. Mol Cancer 2019;18:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, et al. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology 2003;38:1188‐1198. [DOI] [PubMed] [Google Scholar]
- 23. Llacuna L, Bárcena C, Bellido‐Martín L, Fernández L, Stefanovic M, Marí M, et al. Growth arrest‐specific protein 6 is hepatoprotective against murine ischemia/reperfusion injury. Hepatology 2010;52:1371‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zagórska A, Través PG, Jiménez‐García L, Strickland JD, Oh J, Tapia FJ, et al. Differential regulation of hepatic physiology and injury by the TAM receptors Axl and Mer. Life Sci Alliance 2020;3:e202000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strasberg SM, Phillips C. Use and dissemination of the brisbane 2000 nomenclature of liver anatomy and resections. Ann Surg 2013;257:377‐382. [DOI] [PubMed] [Google Scholar]
- 26. Dindo D, Demartines N, Clavien P‐A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rahbari NN, Garden OJ, Padbury R, Brooke‐Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713‐724. [DOI] [PubMed] [Google Scholar]
- 28. Pulford K, Micklem K, McCarthy S, Cordell J, Jones M, Mason DY. A monocyte/macrophage antigen recognized by the four antibodies GHI/61, Ber‐MAC3, Ki‐M8 and SM4. Immunology 1992;75:588‐595. [PMC free article] [PubMed] [Google Scholar]
- 29. Nielsen MC, Hvidbjerg Gantzel R, Clària J, Trebicka J, Møller HJ, Grønbæk H. Macrophage activation markers, CD163 and CD206, in acute‐on‐chronic liver failure. Cells 2020;9:1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Starlinger P, Pereyra D, Haegele S, Braeuer P, Oehlberger L, Primavesi F, et al. Perioperative von Willebrand factor dynamics are associated with liver regeneration and predict outcome after liver resection. Hepatology 2018;67:1516‐1530. [DOI] [PubMed] [Google Scholar]
- 31. Bellan M, Pogliani G, Marconi C, Minisini R, Franzosi L, Alciato F, et al. Gas6 as a putative noninvasive biomarker of hepatic fibrosis. Biomark Med 2016;10:1241‐1249. [DOI] [PubMed] [Google Scholar]
- 32. Ju C, Reilly TP, Bourdi M, Radonovich MF, Brady JN, George JW, et al. Protective role of kupffer cells in acetaminophen‐induced hepatic injury in mice. Chem Res Toxicol 2002;15:1504‐1513. [DOI] [PubMed] [Google Scholar]
- 33. Jin X, Zimmers TA, Perez EA, Pierce RH, Zhang Z, Koniaris LG. Paradoxical effects of short‐ and long‐term interleukin‐6 exposure on liver injury and repair. Hepatology 2006;43:474‐484. [DOI] [PubMed] [Google Scholar]
- 34. Bourdi M, Masubuchi Y, Reilly TP, Amouzadeh HR, Martin JL, George JW, et al. Protection against acetaminophen‐induced liver injury and lethality by interleukin 10: role of inducible nitric oxide synthase. Hepatology 2002;35:289‐298. [DOI] [PubMed] [Google Scholar]
- 35. Yee SB, Bourdi M, Masson MJ, Pohl LR. Hepatoprotective role of endogenous interleukin‐13 in a murine model of acetaminophen‐induced liver disease. Chem Res Toxicol 2007;20:734‐744. [DOI] [PubMed] [Google Scholar]
- 36. Filardy AA, Pires DR, Nunes MP, Takiya CM, Freire‐de‐Lima CG, Ribeiro‐Gomes FL, et al. Proinflammatory clearance of apoptotic neutrophils induces an IL‐12lowIL‐10high regulatory phenotype in macrophages. J Immunol 2010;185:2044‐2050. [DOI] [PubMed] [Google Scholar]
- 37. Marchetti V, Yanes O, Aguilar E, Wang M, Friedlander D, Moreno S, et al. Differential macrophage polarization promotes tissue remodeling and repair in a model of ischemic retinopathy. Sci Rep 2011;1:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sulahian TH, Högger P, Wahner AE, Wardwell K, Goulding NJ, Sorg C, et al. Human monocytes express CD163, which is upregulated by IL‐10 and identical to p155. Cytokine 2000;12:1312‐1321. [DOI] [PubMed] [Google Scholar]
- 39. Buechler C, Ritter M, Orsó E, Langmann T, Klucken J, Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro‐and antiinflammatory stimuli. J Leukoc Biol 2000;67:97‐103. [PubMed] [Google Scholar]
- 40. Högger P, Sorg C. Soluble CD163 inhibits phorbol ester‐induced lymphocyte proliferation. Biochem Biophys Res Comm 2001;288:841‐843. [DOI] [PubMed] [Google Scholar]
- 41. Lee Y‐J, Lee S‐H, Youn Y‐S, Choi J‐Y, Song K‐S, Cho M‐S, et al. Preventing cleavage of Mer promotes efferocytosis and suppresses acute lung injury in bleomycin treated mice. Toxicol Appl Pharmacol 2012;263:61‐72. [DOI] [PubMed] [Google Scholar]
- 42. Alciato F, Sainaghi PP, Sola D, Castello L, Avanzi GC. TNF‐α, IL‐6, and IL‐1 expression is inhibited by GAS6 in monocytes/macrophages. J Leukoc Biol 2010;87:869‐875. [DOI] [PubMed] [Google Scholar]
- 43. Sharif MN, Šošić D, Rothlin CV, Kelly E, Lemke G, Olson EN, et al. Twist mediates suppression of inflammation by type I IFNs and Axl. J Exp Med 2006;203:1891‐1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nepal S, Tiruppathi C, Tsukasaki Y, Farahany J, Mittal M, Rehman J, et al. STAT6 induces expression of Gas6 in macrophages to clear apoptotic neutrophils and resolve inflammation. Proc Natl Acad Sci U S A 2019;116:16513‐16518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zizzo G, Hilliard BA, Monestier M, Cohen PL. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol 2012;189:3508‐3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lafdil F, Chobert MN, Couchie D, Brouillet A, Zafrani ES, Mavier P, et al. Induction of Gas6 protein in CCl4‐induced rat liver injury and anti‐apoptotic effect on hepatic stellate cells. Hepatology 2006;44:228‐239. [DOI] [PubMed] [Google Scholar]
- 47. Couchie D, Lafdil F, Martin‐Garcia N, Laperche Y, Zafrani ES, Mavier P. Expression and role of Gas6 protein and of its receptor Axl in hepatic regeneration from oval cells in the rat. Gastroenterology 2005;129:1633‐1642. [DOI] [PubMed] [Google Scholar]
- 48. Volkmann X, Anstaett M, Hadem J, Stiefel P, Bahr MJ, Lehner F, et al. Caspase activation is associated with spontaneous recovery from acute liver failure. Hepatology 2008;47:1624‐1633. [DOI] [PubMed] [Google Scholar]
- 49. Adebayo D, Morabito V, Andreola F, Pieri G, Luong T‐V, Dhillon A, et al. Mechanism of cell death in acute‐on‐chronic liver failure: a clinico‐pathologic‐biomarker study. Liver Int 2015;35:2564‐2574. [DOI] [PubMed] [Google Scholar]
- 50. Kramer G, Erdal H, Mertens HJMM, Nap M, Mauermann J, Steiner G, et al. Differentiation between cell death modes using measurements of different soluble forms of extracellular cytokeratin 18. Can Res 2004;64:1751‐1756. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Supplementary Material


