Abstract
Australia was one of the first countries with unrestricted access to government subsidized direct‐acting antiviral (DAA) therapy for adults with chronic hepatitis C virus. This study assessed real‐world DAA treatment outcomes across a diverse range of Australian clinical services and evaluated factors associated with successful treatment and loss to follow‐up. Real‐world Effectiveness of Antiviral therapy in Chronic Hepatitis C (REACH‐C) consisted a national observational cohort of 96 clinical services including specialist clinics and less traditional settings such as general practice. Data were obtained on consecutive individuals who commenced DAAs from March 2016 to June 2019. Effectiveness was assessed by sustained virological response ≥12 weeks following treatment (SVR) using intention‐to‐treat (ITT) and per‐protocol (PP) analyses. Within REACH‐C, 10,843 individuals initiated DAAs (male 69%; ≥50 years 52%; cirrhosis 22%). SVR data were available in 85% (9,174 of 10,843). SVR was 81% (8,750 of 10,843) by ITT and 95% (8,750 of 9,174) by PP. High SVR (≥92%) was observed across all service types and participant characteristics. Male gender (adjusted odds ratio [aOR] 0.56, 95% confidence interval [CI] 0.43‐0.72), cirrhosis (aOR 0.52, 95% CI 0.41‐0.64), recent injecting drug use (IDU; aOR 0.64, 95% CI 0.46‐0.91) and previous DAA treatment (aOR 0.50, 95% CI 0.28‐0.90) decreased the likelihood of achieving SVR. Multiple factors modified the likelihood of loss to follow‐up including IDU ± opioid agonist therapy (OAT; IDU only: aOR 1.75, 95% CI 1.44‐2.11; IDU + OAT: aOR 1.39, 95% CI 1.11‐1.74; OAT only, aOR 1.36; 95% CI 1.13‐1.68) and age (aOR 0.97, 95% CI 0.97‐0.98). Conclusion: Treatment response was high in a diverse population and through a broad range of services following universal access to DAA therapy. Loss to follow‐up presents a real‐world challenge. Younger people who inject drugs were more likely to disengage from care, requiring innovative strategies to retain them in follow‐up.

Abbreviations
- aOR
adjusted odds ratio
- CI
confidence interval
- DAA
direct‐acting antiviral
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- IDU
injecting drug use
- IQR
interquartile range
- ITT
intention‐to‐treat
- OAT
opioid agonist therapy
- PBS
pharmaceutical benefits scheme
- PP
per‐protocol
- REACH‐C
Real‐world Effectiveness of Antiviral therapy in Chronic Hepatitis C
- SVR
sustained virological response
Direct‐acting antiviral (DAA) agents for hepatitis C virus (HCV) infection have revolutionized the assessment and management of individuals living with HCV. In the era of interferon‐based therapy, treatment uptake was low due to toxicity and suboptimal efficacy.( 1 ) Many people with HCV were unable to access treatment due to psychiatric disease, drug and alcohol use, advanced liver disease, or other comorbidities.( 2 ) In addition, prescription of interferon‐based treatment was generally limited to gastroenterologists, hepatologists, and infectious diseases physicians within specialist tertiary clinics.
In March 2016, Australia became one of the first countries globally to provide unrestricted access to government‐subsidized DAA therapy for adults living with chronic HCV, driving progress toward HCV elimination. Key features of the subsidized program include no restrictions on liver disease stage or drug and alcohol use.( 3 ) Prescribing of DAAs is also permitted by any registered medical practitioner, including general practitioners, although specialist approval is required for new prescribers. Diagnostic testing, available at no cost to the consumer, is recommended for any individual with exposure risk or HCV‐related disease symptoms.( 4 ) As most prevalent HCV infections (≥80%) in Australia are among people with a history of injecting drug use, screening efforts remain focused on this population.( 4 , 5 ) The broadening of treatment eligibility and prescribing rights facilitated an unprecedented rise in HCV treatment initiations, from 4,740 during 2015 to 32,560 during 2016.( 3 )
While high rates of treatment scale‐up have been observed across North America and Europe, access to treatment has often been restricted by liver disease stage, ongoing substance use, and/or requirement for specialist initiation.( 3 ) The ease of access in Australia, coupled with low toxicity and high efficacy of DAAs, has resulted in many prescriptions occurring outside traditional treatment settings. During the first 2 years of universal access, 38% of individuals received their prescription from a nonspecialist.( 3 ) Consequently, significant uptake has been observed in marginalized populations who traditionally encounter barriers engaging in specialist HCV services, including people who inject drugs and prisoners.( 6 )
With sustained virological response (SVR) consistently above 90% in clinical trials,( 7 , 8 ) emerging data from real‐world cohorts demonstrate equally impressive efficacy in clinical practice.( 9 ) This includes settings with unrestricted access to DAAs such as Australia and Georgia; however, these generally report on select jurisdictions,( 10 , 11 , 12 ) service types,( 13 , 14 , 15 , 16 ) or treatment regimens.( 17 ) Loss to follow‐up within these diverse cohorts varies from 4% to 33%,( 11 , 13 , 15 , 17 ) highlighting a challenge with engaging individuals in posttreatment care. The evaluation of real‐world treatment outcomes and understanding characteristics of individuals who do not return for follow‐up in the Australian context is critical to guide future development and implementation of HCV services.
The primary aim of this analysis was to assess real‐world DAA treatment outcomes across a diverse range of Australian clinical services. This analysis also aimed to evaluate factors associated with successful HCV treatment and loss to follow‐up following treatment initiation.
Participants and Methods
Study Design and Setting
The Real‐world Effectiveness of Antiviral therapy in Chronic Hepatitis C (REACH‐C) study consisted of an observational cohort from an Australian network of 96 diverse clinical services delivered through 33 centers (Supporting Table S1). Services were located across all Australian states and territories, including metropolitan and rural areas. Types of clinical services included specialist liver clinics, general practice, sexual health services, community health clinics, drug and alcohol centers, prisons, telehealth, outreach, Aboriginal and Torres Strait Islander (hereafter referred to as indigenous) health services, and mental health services.
Participants
Data on consecutive patients within the network commencing Australian Government subsidized DAA therapy, through the Pharmaceutical Benefits Scheme (PBS), for chronic HCV infection between March 1, 2016, and June 30, 2019, was collected. Five DAA regimens were initially PBS‐listed on March 1, 2016, with other regimens subsequently approved (Supporting Table S2). To receive PBS‐subsidized treatment during this period, individuals must have been at least 18 years of age and have met the eligibility criteria outlined in the General Statement for Drugs for the Treatment of Hepatitis C.( 18 )
There were no additional eligibility requirements for REACH‐C and no exclusion criteria. The choice of regimen and duration of treatment was at the discretion of the treating clinician, as individuals were treated as part of routine care.
The individuals in REACH‐C were followed as per local procedures of each clinical service, all of which attempted to facilitate HCV‐RNA testing at least 12 weeks following treatment. For individuals who did not return for follow‐up, extensive efforts were made by clinic staff to facilitate testing. Strategies included communication via phone, mail, email and SMS, contact with family and friends, mailing pathology forms, liaising with other health services, organizing patient transport, and opportunistic testing. Individuals could be followed up for treatment outcome data, regardless of treatment commencement date, until the study ended.
Variables
Data on all consecutive individuals initiating DAAs at each service were collected at treatment initiation and at SVR assessment through a combination of retrospective and prospective means (primarily review of medical records or clinic databases). Data variables captured at treatment initiation included demographics (gender, age, indigenous identification), recent injecting drug use (IDU; within prior 6 months), clinical characteristics (HCV genotype, human immunodeficiency virus [HIV] status, hepatitis B virus surface antigen status, cirrhosis status (defined by FibroScan or aspartate aminotransferase–to–platelet ratio index), median liver stiffness measurement by transient elastography (FibroScan), HCV treatment history, current opioid agonist therapy (OAT)), treatment setting (service attended and prescriber type), and prescribed treatment (regimen, duration, and date of initiation). Treatment outcome data (SVR result and reason for not achieving SVR [if applicable]) were collected until June 30, 2020.
Effectiveness of treatment was determined by the proportion of individuals who achieved SVR, defined as undetectable HCV RNA at least 12 weeks after treatment. Virologic failure was defined as detectable HCV RNA at 12 weeks following treatment with exclusion of reinfection. Reinfection was classified as detectable HCV RNA at SVR assessment with an HCV strain distinct from the primary infecting strain (identified by posttreatment genotype switch or sequencing). Participants were classified as being lost to follow‐up if they had an unknown SVR due to no documented HCV RNA test at least 12 weeks after treatment (not attending clinic for follow‐up testing or death).
Statistical Analysis
Categorical parameters were summarized as number and proportion. Continuous variables were summarized by either mean and SD or median and interquartile range (IQR), as appropriate.
Analysis of treatment outcomes used two approaches:
Per protocol (PP): included individuals who commenced DAA treatment and underwent assessment for virological response at least 12 weeks after treatment; and
Intention‐to‐treat (ITT): included all individuals who commenced DAA treatment, including those who were lost to follow‐up during or following treatment, died, or with an unknown SVR (assessed as not achieving SVR).
Logistic regression analysis was used to identify factors associated with SVR (PP population) and loss to follow‐up (ITT population). Potential predictors were determined a priori and included demographic (age, gender, and indigenous identification), clinical (prior HCV treatment, cirrhosis, HIV status, and current OAT), behavioral (recent IDU), type of service initiating treatment, and year of treatment initiation. The adjusted multivariate logistic regression models included variables with P ≤ 0.2 in unadjusted analysis. All statistical tests were two‐sided with a significance level of P < 0.05.
To account for potential unmeasured confounders introduced by clinics initiating HCV treatment, the logistic regression model for loss to follow‐up were adjusted for intraclass correlation, considering each clinical service as a class. Analysis was performed using STATA (version 14.0; Stata Corporation, College Station, TX).
Ethical Approval
Ethical approval for the REACH‐C study was obtained from St. Vincent’s Hospital Sydney Human Research Ethics Committee (HREC/16/SVH/223), Aboriginal Health and Medical Research Council (1280/17), Northern Territory Department of Health and Menzies School of Health Research Human Research Ethics Committee (2018‐3118), Central Australian Human Research Ethics Committee (CA‐18‐3172), Western Australian Aboriginal Health Ethics Committee, Kimberley Aboriginal Health Planning Forum (2018‐008), and Tasmanian Health and Medical Research Ethics Committee (H0017728). Further approvals at local health district levels were acquired for public sites. As this was an audit study, individual consent was not required.
Results
Baseline Characteristics
From March 1, 2016, to June 30, 2019, 10,843 individuals commenced DAA therapy within the REACH‐C network (Table 1). This represents 14% (10,849/76,830) of individuals estimated to have initiated baseline DAA therapy through the Australian PBS scheme.( 19 ) Most were male (69%) and HCV genotype 1 (53%) or genotype 3 (40%). Cirrhosis was reported in 22%, HIV coinfection in 4%, and IDU ± OAT in 16%. Age at commencement of DAA therapy ranged from 18 to 93 years, with a median of 52 years (IQR = 42‐58).
TABLE 1.
Enrollment Demographic and Clinical Characteristics, Overall and by Service Type
| Characteristic | All Patients (n = 10,843) | Specialist Liver Clinic (n = 5,762) | General Practice (n = 2,072) | Sexual Health Service (n = 265) | Community Health Clinic (n = 955) | Drug and Alcohol Service (n = 614) | Prison (n = 679) | Other* (n = 413) |
|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||
| Male | 7,484 (69) | 3,871 (67) | 1,376 (66) | 194 (73) | 671 (70) | 465 (68) | 623 (92) | 275 (67) |
| Female | 3,341 (31) | 1,883 (33) | 696 (34) | 69 (26) | 278 (29) | 219 (32) | 56 (8) | 137 (33) |
| Transgender | 18 (<1) | 8 (<1) | 0 (0) | 2 (1) | 6 (1) | 1 (<1) | 0 (0) | 1 (<1) |
| Age, median (IQR) | 52 (42‐58) | 54 (46‐59) | 50 (42‐57) | 48 (38‐56) | 47 (40‐54) | 46 (40‐54) | 37 (30‐43) | 50 (40‐57) |
| ≥50 years | 5,677 (52) | 3,615 (63) | 1,062 (51) | 118 (45) | 366 (38) | 249 (36) | 62 (9) | 198 (48) |
| <50 years | 5,166 (48) | 2,147 (37) | 1,010 (49) | 147 (55) | 589 (62) | 435 (64) | 617 (91) | 215 (52) |
| Indigenous identification | ||||||||
| Yes | 915 (8) | 245 (4) | 168 (8) | 20 (8) | 198 (21) | 37 (5) | 182 (27) | 65 (16) |
| No | 8,096 (75) | 4,690 (81) | 1,246 (60) | 232 (88) | 732 (76) | 472 (69) | 419 (62) | 303 (73) |
| Unknown | 1,832 (17) | 827 (15) | 658 (31) | 13 (5) | 25 (3) | 176 (26) | 78 (12) | 45 (11) |
| Genotype | ||||||||
| 1a | 4,402 (41) | 2,338 (41) | 877 (42) | 126 (48) | 359 (38) | 263 (39) | 269 (40) | 164 (40) |
| 1b | 857 (8) | 513 (9) | 192 (9) | 20 (8) | 61 (6) | 38 (6) | 16 (2) | 16 (4) |
| 1, not specified | 407 (4) | 229 (4) | 67 (3) | 9 (3) | 31 (3) | 26 (4) | 29 (4) | 16 (4) |
| 2 | 463 (4) | 273 (5) | 85 (4) | 7 (3) | 38 (4) | 33 (5) | 10 (1) | 17 (4) |
| 3 | 4,352 (40) | 2,193 (38) | 814 (39) | 92 (35) | 430 (45) | 297 (44) | 334 (49) | 187 (45) |
| 4 | 106 (1) | 79 (1) | 5 (<1) | 4 (2) | 10 (1) | 3 (<1) | 4 (1) | 1 (<1) |
| 5 | 2 (<1) | 2 (<1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 6 | 108 (1) | 79 (1) | 8 (<1) | 2 (1) | 6 (1) | 4 (1) | 5 (1) | 4 (1) |
| Mixed | 50 (<1) | 31 (1) | 9 (<1) | 1 (<1) | 1 (<1) | 2 (<1) | 5 (1) | 2 (1) |
| Unknown | 96 (1) | 25 (1) | 15 (1) | 4 (1) | 19 (2) | 19 (3) | 8 (1) | 6 (1) |
| Stage of liver disease † | ||||||||
| No or mild fibrosis (F0‐F1) | 3,805 (35) | 1,878 (33) | 842 (41) | 115 (43) | 479 (50) | 281 (41) | 126 (19) | 81 (20) |
| Moderate fibrosis (F2) | 1,224 (11) | 602 (11) | 266 (13) | 37 (14) | 152 (16) | 69 (10) | 48 (7) | 30 (7) |
| Advanced fibrosis (F3) | 745 (7) | 465 (8) | 103 (5) | 26 (10) | 74 (8) | 47 (7) | 13 (2) | 17 (4) |
| Cirrhosis (F4) | 1,549 (14) | 1,102 (19) | 144 (7) | 37 (14) | 115 (12) | 83 (12) | 17 (3) | 49 (11) |
| FibroScan not performed | 3,519 (32) | 1,695 (29) | 717 (35) | 50 (19) | 135 (14) | 205 (30) | 475 (57) | 236 (57) |
| Cirrhosis ‡ | ||||||||
| Yes | 2,353 (22) | 1,661 (29) | 214 (10) | 47 (17) | 127 (13) | 125 (18) | 59 (9) | 117 (28) |
| No | 8,438 (78) | 4,087 (71) | 1,848 (89) | 216 (82) | 820 (86) | 552 (81) | 616 (91) | 290 (70) |
| Unknown | 52 (<1) | 13 (<1) | 10 (<1) | 2 (1) | 8 (1) | 8 (1) | 4 (1) | 6 (2) |
| IDU/OAT | ||||||||
| IDU + OAT | 984 (9) | 328 (6) | 101 (5) | 32 (12) | 276 (29) | 21 (3) | 165 (24) | 60 (15) |
| IDU only | 786 (7) | 171 (3) | 71 (3) | 22 (8) | 204 (21) | 204 (21) | 34 (5) | 35 (8) |
| OAT only | 1151 (11) | 393 (7) | 289 (14) | 25 (9) | 71 (7) | 260 (38) | 79 (11) | 32 (8) |
| Neither | 6,074 (56) | 3,981 (69) | 1,199 (58) | 63 (24) | 339 (36) | 78 (11) | 172 (25) | 237 (57) |
| Unknown | 1,848 (17) | 889 (15) | 412 (20) | 123 (46) | 65 (7) | 77 (11) | 229 (33) | 49 (12) |
| Comorbidities | ||||||||
| HIV | 398 (4) | 222 (4) | 45 (2) | 95 (36) | 23 (2) | 9 (1) | 3 (<1) | 0 (<1) |
| HBV | 123 (1) | 67 (1) | 10 (<1) | 6 (2) | 17 (2) | 4 (1) | 14 (2) | 5 (1) |
| Previous HCV treatment and regimen | ||||||||
| No | 9,329 (86) | 4,753 (82) | 1,872 (90) | 222 (84) | 876 (92) | 623 (91) | 616 (91) | 355 (86) |
| Yes, interferon‐containing | 1,249 (12) | 906 (16) | 165 (8) | 24 (9) | 45 (5) | 33 (5) | 40 (6) | 36 (9) |
| Yes, interferon‐free | 156 (1) | 84 (1) | 14 (1) | 3 (1) | 19 (2) | 9 (1) | 16 (2) | 11 (3) |
| Yes, unknown regimen | 109 (1) | 19 (<1) | 21 (1) | 16 (6) | 15 (2) | 20 (3) | 7 (1) | 11 (3) |
| Prescribed therapy | ||||||||
| SOF/LDV | 4,220 (39) | 2,347 (41) | 878 (42) | 125 (47) | 267 (28) | 250 (37) | 215 (32) | 132 (32) |
| SOF/DCV | 3,061 (28) | 1,756 (30) | 498 (24) | 97 (37) | 213 (22) | 195 (28) | 196 (29) | 103 (25) |
| SOF + RBV | 222 (2) | 159 (3) | 31 (2) | 3 (1) | 11 (1) | 9 (1) | 5 (1) | 4 (1) |
| PrOD | 111 (1) | 86(1) | 14 (1) | 0 (0) | 5 (1) | 3 (<1) | 1 (<1) | 2 (<1) |
| GRZ/ELB | 452 (4) | 244 (4) | 80 (4) | 7 (3) | 68 (7) | 19 (3) | 26 (4) | 9 (2) |
| GRZ/ELB + SOF | 11 (<1) | 11 (<1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| SOF/VEL | 1,257 (21) | 1,001 (17) | 471 (23) | 33 (12) | 307 (32) | 159 (23) | 134 (32) | 134 (32) |
| SOF/VEL/VOX | 26 (<1) | 13 (<1) | 6 (<1) | 0 (0) | 3 (<1) | 1 (<1) | 1 (<1) | 2 (<1) |
| GLE/PIB | 433 (4) | 130 (2) | 88 (4) | 0 (0) | 72 (8) | 44 (6) | 78 (12) | 21 (5) |
| Prescribed treatment duration | ||||||||
| 8 weeks | 1,437 (13) | 457 (8) | 458 (22) | 36 (14) | 145 (15) | 107 (16) | 190 (28) | 6 (<1) |
| 12 weeks | 8,128 (75) | 4,385 (76) | 1,527 (75) | 193 (73) | 740 (78) | 521 (76) | 454 (67) | 302 (73) |
| 16 weeks | 20 (<1) | 17 (<1) | 0 (0) | 0 (0) | 2 (<1) | 0 (0) | 1 (<1) | 0 (0) |
| 24 weeks | 1,218 (11) | 896 (16) | 80 (4) | 36 (14) | 55 (6) | 53 (8) | 21 (5) | 65 (16) |
| Location of health service provision | ||||||||
| Major city | 6,945 (64) | 4,088 (71) | 1,063 (51) | 120 (45) | 750 (79) | 585 (85) | 280 (41) | 58 (14) |
| Regional or remote | 3,898 (36) | 1,674 (29) | 1,009 (49) | 145 (55) | 205 (21) | 100 (15) | 399 (59) | 355 (86) |
Data are presented as n (%) unless otherwise specified.
Outreach, telehealth, mental health, and indigenous health services.
Determined by FibroScan.
Determined by any method.
Abbreviations: DCV, daclatasvir; ELB, elbasvir; GLE, glecaprevir; GZR, grazoprevir; LDV, ledipasvir; PIB, pibrentasvir; PrOD, paritaprevir/ritonavir/ombitasvir + dasabuvir; RBV, ribavirin; SOF, sofosbuvir; VEL, velpatasvir; VOX, voxilaprevir.
Treatment was most commonly initiated through specialist liver clinics (53%), general practice (19%), or community health clinics (9%). DAAs were primarily prescribed by gastroenterologists/hepatologists (38%), infectious disease physicians (25%), and general practitioners (24%). Specialists, inclusive of gastroenterologists/hepatologists and infectious disease physicians, initiated treatment in a higher proportion with cirrhosis (27%) compared with general practitioners (10%) (Table 2).
TABLE 2.
Enrollment Demographic and Clinical Characteristics by Prescriber Type
| Characteristic | Specialist† (n = 6,925) | General Practitioner (n = 2,558) | Other † (n = 1,340) |
|---|---|---|---|
| Cirrhosis ‡ | |||
| Yes | 1,853 (27) | 251 (10) | 246 (18) |
| No | 5,053 (73) | 2,281 (89) | 1,088 (81) |
| Unknown | 19 (<1) | 26 (1) | 6 (<1) |
| Prescribed therapy | |||
| SOF/LDV | 2,693 (39) | 1,023 (40) | 496 (37) |
| SOF/DCV | 2,064 (30) | 619 (24) | 375 (28) |
| SOF+RBV | 167 (2) | 38 (1) | 17 (1) |
| PrOD | 82 (1) | 16 (1) | 13 (1) |
| GRZ/ELB | 277 (4) | 95 (4) | 80 (6) |
| GRZ/ELB+SOF | 11 (<1) | 0 (0) | 0 (0) |
| SOF/VEL | 1,365 (20) | 633 (25) | 253 (19) |
| SOF/VEL/VOX | 16 (<1) | 6 (<1) | 4 (<1) |
| GLE/PIB | 227 (3) | 105 (4) | 101 (8) |
| Prescribed treatment duration | |||
| 8 weeks | 637 (9) | 542 (21) | 254 (19) |
| 12 weeks | 5,246 (76) | 1,898 (74) | 972 (73) |
| 16 weeks | 15 (<1) | 0 (0) | 5 (<1) |
| 24 weeks | 1,014 (15) | 96 (4) | 106 (8) |
Data are presented as n (%).
Gastroenterologist, hepatologist, or infectious disease physician.
Sexual health physician, general physician, mental health practitioner, nurse practitioner, or other.
Determined by any method.
Abbreviations: DCV, daclatasvir; ELB, elbasvir; GLE, glecaprevir; GZR, grazoprevir; LDV, ledipasvir; PIB, pibrentasvir; PrOD, paritaprevir/ritonavir/ombitasvir + dasabuvir; RBV, ribavirin; SOF, sofosbuvir; VEL, velpatasvir; VOX, voxilaprevir.
When comparing treatment initiations from March 2016 to June 2019, there was a decline over time in the proportion of individuals initiated by specialists (80% vs. 42%). The proportion receiving prescriptions from a general practitioner increased from 12% in March 2016 to 27% in June 2019.
The proportion of treatment initiations in people aged ≥50 years declined over time, from 59% in 2016 to 44% in 2019 (Table 3) ± OAT. Initiations by gender remained stable over time, with 69% male in 2016 compared with 71% in 2019. From 2016 to 2019, there was an increase in the proportion initiated in prison (4% vs. 10%).
TABLE 3.
Enrollment Demographic and Clinical Characteristics by Year of Treatment Initiation
| Characteristic | 2016 (n = 5,341) | 2017 (n = 3,111) | 2018 (n = 1,831) | 2019 (n = 560) |
|---|---|---|---|---|
| Gender | ||||
| Male | 3,686 (69) | 2,128 (68) | 1,271 (69) | 39 (71) |
| Female | 1,649 (31) | 978 (31) | 553 (30) | 161 (29) |
| Transgender | 6 (<1) | 5 (<1) | 7 (<1) | 0 (0) |
| Age | ||||
| ≥50 years | 3,147 (59) | 1,508 (48) | 773 (42) | 249 (44) |
| <50 years | 2,194 (41) | 1,603 (52) | 1,058 (58) | 311 (56) |
| Indigenous identification | ||||
| Yes | 315 (6) | 280 (9) | 242 (13) | 78 (14) |
| No | 4,185 (78) | 2,250 (72) | 1,263 (69) | 398 (71) |
| Unknown | 841 (16) | 581 (19) | 326 (18) | 84 (15) |
| Cirrhosis | ||||
| Yes | 1,280 (24) | 630 (21) | 343 (19) | 100 (18) |
| No | 4,054 (76) | 2,463 (79) | 1,471 (80) | 450 (80) |
| Unknown | 7 (<1) | 18 (1) | 17 (1) | 10 (2) |
| IDU/OAT | ||||
| IDU + OAT | 266 (5) | 231 (7) | 211 (12) | 78 (14) |
| IDU only | 284 (5) | 329 (11) | 270 (15) | 101 (18) |
| OAT only | 588 (11) | 342 (11) | 172 (9) | 49 (9) |
| Neither | 3,087 (58) | 1,760 (57) | 949 (52) | 278 (50) |
| Unknown | 1,116 (21) | 449 (14) | 229 (13) | 54 (10) |
| Location of health service provision | ||||
| Major city | 3,358 (63) | 1,936 (62) | 1,255 (69) | 396 (71) |
| Regional or remote | 1,983 (37) | 1,175 (38) | 576 (31) | 164 (29) |
Data are presented as n (%) unless otherwise specified.
The most commonly prescribed regimen was sofosbuvir/ledipasvir (39%), followed by sofosbuvir + daclatasvir (28%). Prescribing patterns evolved with the introduction of additional regimens to the PBS. The proportion of individuals prescribed sofosbuvir/ledipasvir declined from 53% in March 2016 to 10% by June 2019. In June 2019, sofosbuvir/velpatasvir (44%) and glecaprevir/pibrentasvir (40%) were the most commonly prescribed regimens.
Overall Treatment Outcomes
Virological data at or following 12 weeks following treatment were available for 9,174 of 10,843 individuals (85%) (Fig. 1). Reasons for missing data included death (79 of 1,669) and not attending for follow‐up testing (1,600 of 1,669).
FIG. 1.
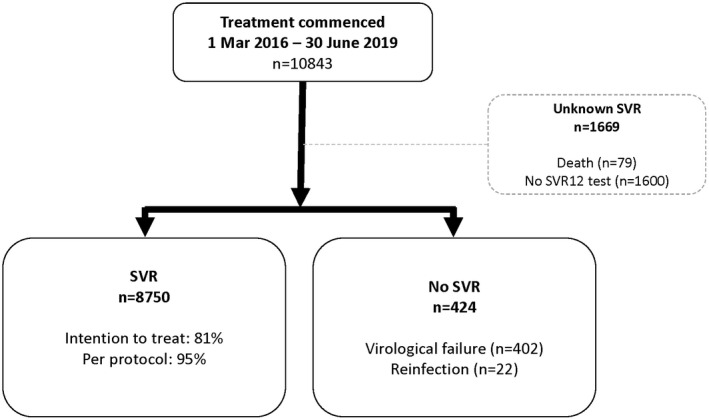
Patient disposition.
By ITT analysis, including the 1,669 patients without SVR data, SVR was 81% (8,750 of 10,843). In those with SVR data (PP population), 8,750 of 9,174 (95%) achieved SVR.
At 12 weeks following treatment, 424 individuals had virological recurrence, of which 402 cases (4% of total cohort) were deemed virological failure and 22 cases (<1% of total cohort) reinfection. Virological failure was highest in genotype 4 (8 of 106; 8%), genotype 2 (24 of 463; 5%), and genotype 3 (225 of 4,352; 5%). Although the proportion of virological failure remained stable at 4% from 2016‐2019, the proportion of unknown SVR increased each year (2016, 9%; 2017, 17%; 2018, 26%; and 2019, 31%) (Fig. 2).
FIG. 2.
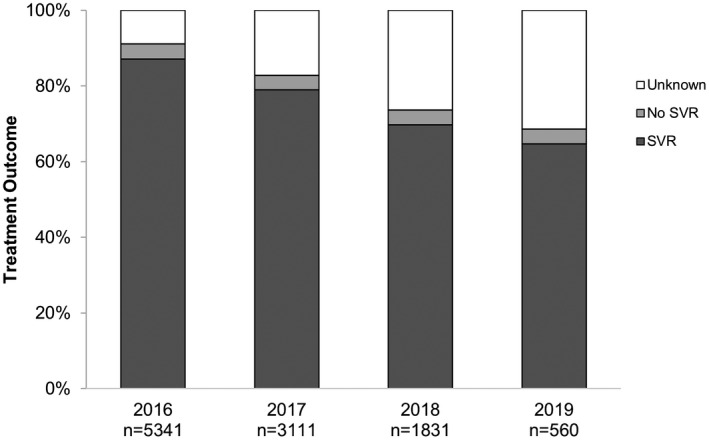
Treatment outcomes by year of DAA initiation.
Factors Associated With Achieving SVR (PP Population)
High SVR (≥92%) were observed across all service types (Supporting Fig. S1) and baseline characteristics (Supporting Fig. S2). By genotype, SVRs were also high (1a, 97%; 1b, 98%; 3, 94%; and 4/5/6, 94%).
Logistic regression was undertaken to determine factors associated with achieving SVR (Table 4). In adjusted analysis, factors independently associated with decreased likelihood of SVR were male gender (adjusted odds ratio [aOR] 0.56; 95% confidence interval [CI] 0.43‐0.72, P < 0.01), cirrhosis (aOR 0.52; 95% CI 0.42‐0.65, P < 0.01), recent IDU (aOR 0.63; 95% CI 0.45‐0.90, P = 0.01), and previous interferon‐free HCV treatment (aOR 0.51; 95% CI 0.28‐0.91, P = 0.02). Factors associated with SVR were receiving OAT in the absence of recent IDU (aOR 1.78; 95% CI 1.16‐2.72, P < 0.01), unknown IDU/OAT (aOR 1.76; 95% CI 1.22‐2.52, P < 0.01), and treatment initiation through a community health clinic (aOR 2.04; 95% CI 1.26‐3.30, P < 0.01). Age, indigenous identification, HIV coinfection, year of treatment initiation, and location of health service provision were not associated with achieving SVR in adjusted analysis.
TABLE 4.
Logistic Regression Analysis of Factors Associated With Achieving SVR in the PP Population
| Variable | Achieved SVR (n = 8,750) | Did Not Achieve SVR (n = 424) | Unadjusted | Adjusted | ||
|---|---|---|---|---|---|---|
| OR | P | OR † | P | |||
| (95% CI) | (95%s CI) | |||||
| Sex | ||||||
| Female (ref) | 2,760 (97) | 82 (3) | — | — | ||
| Male | 5,976 (95) | 342 (5) | 0.52 (0.41‐0.66) | <0.01 | 0.56 (0.43‐0.72) | <0.01 |
| Transgender | 14 (100) | 0 (0) | — | |||
| Age, median (IQR) | ||||||
| Age in years | 52 (43‐58) | 53 (44‐59) | 1.04 (1.03‐1.04) | <0.01 | 1.00 (0.99‐1.01) | 0.73 |
| Age | ||||||
| ≥60 (ref) | 1,563 (95) | 88 (5) | — | |||
| 50‐59 | 3,275 (95) | 158 (5) | 1.17 (0.89‐1.52) | 0.26 | ||
| 40‐49 | 2,221 (96) | 104 (4) | 1.20 (0.90‐1.61) | 0.22 | ||
| 30‐39 | 1,332 (96) | 49 (4) | 1.53 (1.01‐2.19) | 0.02 | ||
| 18‐29 | 359 (93) | 25 (7) | 0.81 (0.51‐1.28) | 0.36 | ||
| Indigenous identification | ||||||
| No (ref) | 6,595 (95) | 327 (5) | — | — | ||
| Yes | 656 (94) | 41 (6) | 0.79 (0.57‐1.10) | 0.18 | 0.78 (0.55‐1.12) | 0.17 |
| Unknown | 1,499 (96) | 56 (4) | 1.33 (0.99‐1.77) | 0.06 | 1.13 (0.83‐1.53) | 0.44 |
| Cirrhosis* | ||||||
| No (ref) | 6,810 (96) | 266 (4) | — | — | ||
| Yes | 1,909 (92) | 158 (8) | 0.47 (0.39‐0.58) | <0.01 | 0.52 (0.42‐0.65) | <0.01 |
| Unknown | 31 (100) | 0 (0) | — | |||
| IDU/OAT | ||||||
| Neither (ref) | 5,108 (95) | 277 (5) | — | — | ||
| IDU only | 669 (93) | 51 (7) | 0.71 (0.52‐0.97) | 0.03 | 0.63 (0.45‐0.90) | 0.01 |
| IDU + OAT | 570 (95) | 32 (5) | 0.97 (0.66‐1.41) | 0.86 | 0.75 (0.49‐1.15) | 0.19 |
| OAT only | 932 (97) | 26 (3) | 1.94 (1.29‐2.92) | <0.01 | 1.78 (1.16‐2.72) | 0.01 |
| Unknown | 1,471 (97) | 38 (3) | 2.10 (1.49‐2.96) | <0.01 | 1.76 (1.22‐2.52) | <0.01 |
| HIV status | ||||||
| Negative (ref) | 7,982 (95) | 397 (5) | — | — | ||
| Positive | 358 (97) | 12 (3) | 1.48 (0.83‐2.66) | 0.19 | 1.25 (0.68‐2.29) | 0.47 |
| Unknown | 410 (96) | 15 (4) | 1.36 (0.80‐2.30) | 0.25 | 1.12 (0.65‐1.91) | 0.69 |
| Previous HCV treatment | ||||||
| No (ref) | 7,469 (96) | 340 (4) | — | — | ||
| Yes, interferon‐free | 115 (89) | 14 (11) | 0.37 (0.21‐0.66) | <0.01 | 0.51 (0.28‐0.91) | 0.02 |
| Yes, interferon‐containing | 1,093 (94) | 69 (6) | 0.72 (0.55‐0.94) | 0.02 | 0.82 (0.62‐1.09) | 0.17 |
| Unknown | 73 (99) | 1 (1) | 3.32 (0.46‐23.98) | 0.23 | 2.99 (0.41‐21.80) | 0.28 |
| Service type | ||||||
| Specialist liver clinic (ref) | 4,875 (95) | 254 (5) | — | — | ||
| General practice | 1,608 (96) | 69 (4) | 1.21 (0.93‐1.59) | 0.16 | 0.98 (0.74‐1.31) | 0.91 |
| Sexual health service | 205 (100) | 1 (<1) | 10.68 (1.49‐76.50) | 0.02 | 8.08 (1.11‐58.72) | 0.04 |
| Community health clinic | 716 (97) | 21 (3) | 1.77 (1.13‐2.79) | 0.13 | 2.04 (1.26‐3.30) | <0.01 |
| Drug and alcohol service | 526 (96) | 20 (4) | 1.37 (0.86‐2.18) | 0.18 | 1.18 (0.70‐1.99) | 0.54 |
| Prison | 515 (94) | 34 (6) | 0.79 (0.55‐1.14) | 0.21 | 0.83 (0.53‐1.29) | 0.40 |
| Other | 297 (92) | 25 (8) | 0.62 (0.40‐0.95) | 0.03 | 0.74 (0.47‐1.17) | 0.20 |
| Unknown | 8 (100) | 0 (0) | — | — | ||
| Location of health service provision | ||||||
| Major city | 5,689 (96) | 245 (4) | — | — | ||
| Regional or remote | 3,240 (94) | 179 (6) | 0.74 (0.60‐0.90) | <0.01 | 0.87 (0.70‐1.08) | 0.21 |
| Year of treatment initiation | ||||||
| 2016 (ref) | 4,654 (96) | 213 (4) | — | — | ||
| 2017 | 2,457 (95) | 118 (4) | 0.95 (0.76‐1.20) | 0.68 | 0.96 (0.76‐1.22) | 0.74 |
| 2018 | 1,277 (95) | 71 (5) | 0.82 (0.62‐1.08) | 0.17 | 0.87 (0.65‐1.17) | 0.36 |
| 2019 | 362 (94) | 22 (6) | 0.75 (0.48‐1.18) | 0.22 | 0.85 (0.53‐1.36) | 0.50 |
Data are presented as n (%) unless otherwise specified.
Determined by any method.
n = 9,121.
Abbreviation: OR, odds ratio.
Factors Associated With Loss to Follow‐up
In adjusted analysis (Table 5), factors independently associated with increased loss to follow‐up were IDU ± OAT (IDU only: aOR 1.76, 95% CI 1.45‐2.14, P < 0.01; IDU + OAT: aOR 1.44, 95% CI 1.15‐1.80, P < 0.01; OAT only: aOR 1.39, 95% CI 1.14‐1.70, P < 0.01; and unknown IDU/OAT: aOR 1.89, 95% CI 1.59‐2.25, P < 0.01) and initiation of treatment after 2016 (2017: aOR 2.16, 95% CI 1.87‐2.49, P < 0.01; 2018: aOR 3.63, 95% CI 3.09‐4.27, P < 0.01; and 2019: aOR 4.49, 95% CI 3.57‐5.64, P < 0.01). Factors associated with decreased likelihood of loss to follow‐up were older age (aOR 0.97, 95% CI 0.97‐0.98, P < 0.01), HIV coinfection (aOR 0.39, 95% CI 0.25‐0.61, P < 0.01), and previous interferon‐containing HCV treatment (aOR 0.58, 95% CI 0.46‐0.73, P < 0.01). Gender, cirrhosis, indigenous identification, service type, and location of health service provision were not associated with loss to follow‐up in adjusted analysis.
TABLE 5.
Logistic Regression Analysis of Factors Associated With Loss to Follow‐up
| Variable | Attended SVR12 (n = 9,174) | Did Not Attend SVR12 (n = 1,669) | Unadjusted | Adjusted | ||
|---|---|---|---|---|---|---|
| OR | P | OR † | P | |||
| (95% CI) | (95% CI) | |||||
| Sex | ||||||
| Female (ref) | 2,842 (85) | 499 (15) | — | — | ||
| Male | 6,318 (84) | 1,166 (16) | 1.01 (0.89‐1.13) | 0.09 | 1.06 (0.93‐1.20) | 0.38 |
| Transgender | 14 (78) | 4 (22) | 1.42 (0.45‐4.56) | 0.55 | 1.28 (0.40‐4.16) | 0.68 |
| Age, median (IQR) | ||||||
| Age in years | 52 (43‐58) | 45 (38‐54) | 0.96 (0.96‐0.97) | <0.01 | 0.97 (0.97‐0.98) | <0.01 |
| Age | ||||||
| ≥60 (ref) | 1651 (92) | 148 (8) | — | |||
| 50‐59 | 3433 (89) | 445 (11) | 1.42 (1.17‐1.74) | <0.01 | ||
| 40‐49 | 2,325 (81) | 531 (19) | 2.43 (1.99‐2.97) | <0.01 | ||
| 30‐39 | 1,381 (77) | 419 (23) | 3.25 (2.64‐4.02) | <0.01 | ||
| 18‐29 | 384 (75) | 126 (25) | 3.45 (2.60‐4.56) | <0.01 | ||
| Indigenous identification | ||||||
| No (ref) | 6,922 (86) | 1,174 (15) | — | — | ||
| Yes | 697 (76) | 218 (24) | 1.70 (1.42‐2.03) | <0.01 | 1.21 (1.00‐1.46) | 0.05 |
| Unknown | 1555 (85) | 277 (15) | 1.19 (0.97‐1.41) | 0.09 | 1.04 (0.86‐1.26) | 0.69 |
| Cirrhosis* | ||||||
| No (ref) | 7,076 (84) | 1,362 (16) | — | — | ||
| Yes | 2,067 (88) | 286 (12) | 0.81 (0.70‐0.94) | <0.01 | 1.04 (0.89‐1.22) | 0.60 |
| Unknown | 31 (60) | 21 (40) | 2.48 (1.37‐4.47) | <0.01 | 1.38 (0.75‐2.55) | 0.31 |
| IDU/OST | ||||||
| Neither (ref) | 5,285 (89) | 689 (11) | — | — | ||
| IDU only | 720 (73) | 264 (27) | 2.52 (2.10‐3.02) | <0.01 | 1.76 (1.45‐2.14) | <0.01 |
| IDU + OAT | 602 (77) | 184 (23) | 2.02 (1.63‐2.51) | <0.01 | 1.44 (1.15‐1.80) | <0.01 |
| OAT only | 958 (83) | 193 (17) | 1.52 (1.26‐1.84) | <0.01 | 1.39 (1.14‐1.70) | <0.01 |
| Unknown | 1,509 (82) | 339 (18) | 1.98 (1.68‐2.33) | <0.01 | 1.89 (1.59‐2.25) | <0.01 |
| HIV status | ||||||
| Negative (ref) | 8,379 (85) | 1,523 (15) | — | — | ||
| Positive | 370 (93) | 28 (7) | 0.38 (0.25‐0.59) | <0.01 | 0.39 (0.25‐0.61) | <0.01 |
| Unknown | 425 (78) | 118 (22) | 1.50 (1.17‐1.89) | <0.01 | 1.23 (0.96‐1.57) | 0.10 |
| Previous HCV treatment | ||||||
| No (ref) | 7,809 (84) | 1,520 (16) | — | — | ||
| Yes – interferon‐free | 129 (83) | 27 (17) | 0.95 (0.62‐1.47) | 0.82 | 0.66 (0.42‐1.05) | 0.08 |
| Yes – interferon‐containing | 1,162 (93) | 87 (7) | 0.42 (0.33‐0.52) | <0.01 | 0.58 (0.46‐0.73) | <0.01 |
| Unknown | 74 (68) | 35 (32) | 1.54 (1.00‐2.37) | 0.05 | 1.18 (0.75‐1.86) | 0.48 |
| Service type | ||||||
| Specialist liver clinic (ref) | 5,129 (89) | 633 (11) | — | — | ||
| GP/SHS/CHC | 2,620 (80) | 672 (20) | 1.97 (1.35‐2.88) | <0.01 | 1.39 (0.95‐2.03) | 0.09 |
| Drug and alcohol service | 546 (80) | 139 (20) | 1.84 (1.14‐2.96) | 0.01 | 1.17 (0.72‐1.90) | 0.52 |
| Prison | 549 (81) | 130 (19) | 1.62 (0.92‐2.87) | 0.10 | 0.59 (0.34‐1.05) | 0.08 |
| Other | 322 (78) | 91 (22) | 2.44 (1.43‐4.14) | <0.01 | 1.28 (0.76‐2.14) | 0.36 |
| Unknown | 8 (67) | 4 (33) | 3.82 (0.76‐19.17) | 0.10 | 3.02 (0.58‐15.64) | 0.19 |
| Location of health service provision | ||||||
| Major city (ref) | 5,934 (85) | 1,011 (15) | — | — | ||
| Regional or remote | 3,240 (83) | 658 (17) | 1.31 (0.93‐1.83) | 0.12 | 1.37 (1.00‐1.88) | 0.05 |
| Year of treatment initiation | ||||||
| 2016 (ref) | 4,867 (91) | 474 (9) | — | — | ||
| 2017 | 2,575 (83) | 536 (17) | 2.32 (2.02‐2.67) | <0.01 | 2.16 (1.87‐2.49) | <0.01 |
| 2018 | 1,348 (74) | 483 (26) | 3.94 (3.37‐4.60) | <0.01 | 3.63 (3.09‐4.27) | <0.01 |
| 2019 | 384 (69) | 176 (31) | 4.74 (3.80‐5.90) | <0.01 | 4.49 (3.57‐5.64) | <0.01 |
Data are presented as n (%) unless otherwise specified.
Determined by any method.
n=10,843.
Abbreviations: CHC, community health center; GP, general practice; SHS, sexual health service.
Discussion
The REACH‐C study is one of the largest real‐world cohorts exploring HCV treatment outcomes through diverse clinical services. Consistently high proportions were cured across clinical settings and population subgroups, supporting Australia’s approach of unrestricted and broad treatment access. Year of treatment initiation was the strongest predictor of loss to follow‐up, with younger people and those reporting recent injecting drug use most likely not to present for SVR testing. With the potential for loss to follow‐up to jeopardize HCV elimination efforts, identification of populations most at risk can inform monitoring and engagement strategies.
High SVR (95%) in the PP population provides evidence of treatment effectiveness, consistent with clinical trial data( 8 ) and other real‐world cohorts.( 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 ) This finding is not only reassuring but incredibly encouraging, given the diversity of both the individuals accessing treatment and the services through which treatment was initiated. In contrast to many other countries, treatment expansion in Australia has not been subject to restrictions on the basis of patient or prescriber characteristics. High cure rates observed across all types of clinical services (≥92%) demonstrated equivalent outcomes between nontraditional models of care and specialist liver clinics. Importantly, the delivery of HCV care through decentralized models has translated to substantial declines in prevalence among key populations with transmission risk.( 20 , 21 , 22 ) Although conducted within a high‐income setting, this study provides important evidence for clinical and public health management of HCV globally, including in low‐income and middle‐income countries where there may be limited access to tertiary services.
In striving for HCV elimination, Australia’s national HCV strategy highlights the need to engage marginalized populations that are typically hard to reach, requiring significant contribution from general practice and community services.( 23 ) Although specialists initiated treatment in nearly two‐thirds of the REACH‐C cohort overall, prescribing patterns evolved over time to become more community‐based, with the proportion of general practitioner initiations more than doubling throughout the study period. In Australia, general practitioners new to DAA prescribing are supported through a number of mechanisms including specialist consultation through pathways such as the REACH‐C online portal( 24 ) and hepatitis C prescriber training provided by the Australasian Society for HIV, Viral Hepatitis, and Sexual Health Medicine and the Gastroenterological Society of Australia. Despite these efforts, the absolute numbers of general practitioners prescribing in Australia has plateaued, and may compromise the goal of HCV elimination by 2030.( 19 ) Complex barriers to DAA prescribing have been identified by primary care practitioners including lack of knowledge, perceptions of HCV as a specialist area, and people with HCV being perceived as a challenge to manage.( 25 , 26 ) Further work needs to be done to improve knowledge and awareness of HCV diagnosis and treatment in general practice.
Although there were demographic, clinical, and behavioral factors associated with achieving SVR, high effectiveness was consistently observed across key subpopulations. Although we were unable to distinguish between compensated and decompensated cirrhosis in the REACH‐C cohort, the association of cirrhosis with treatment failure is consistent with previous reports. Findings from a pooled analysis of clinical practice cohorts across North America and Europe treated with sofosbuvir/velpatasvir (n = 5,552) reported that those with compensated cirrhosis were 3 times more likely (odds ratio = 2.53) not to achieve SVR than those without cirrhosis.( 27 ) Decompensated cirrhosis has been similarly described as a negative predictor of SVR in other international cohorts.( 28 , 29 , 30 ) The observation that IDU reduced response to treatment in REACH‐C has been previously described by the HCV‐HIV Transatlantic Research Network, a cohort of people with HCV‐HIV coinfection.( 31 ) In the present study, OAT in combination with IDU appeared to be protective, as SVR was not reduced in this subgroup. Additionally, OAT in the absence of IDU was associated with higher treatment effectiveness, suggesting a more stable population and highlighting a valuable role of OAT programs and drug health services among people who inject drugs. It should be noted that as REACH‐C did not capture treatment adherence, its interaction with SVR in these populations is unclear. Despite statistical differences in SVR when comparing some subpopulations, the differences are generally small (e.g., 95% in men vs. 97% in women) and unlikely to be clinically relevant, given the universally high response.
Although DAAs are proven to be highly efficacious, loss to follow‐up usually exceeds virological failure and has the potential to impact real‐world effectiveness. Overall, 15% of the REACH‐C cohort were lost to follow‐up, although the proportion varied significantly across a range of demographic, clinical, and behavioral factors. The retrospective British Columbia Hepatitis Testers Cohort (n = 4,144) reported lower loss to follow‐up (10%) among genotype 1 and 3 infected individuals,( 32 ) in contrast to higher loss to follow‐up (31%) in people receiving sofosbuvir‐based regimens in Georgia. Loss to follow‐up in real‐world studies is typically higher than in clinical trials, as trial participants are often highly selected and less likely to have high‐risk injecting behaviors, mental health disorders, unstable housing, or incarceration.( 22 , 33 , 34 ) The comparison of loss to follow‐up across real‐world cohorts is challenging due to high variability in definitions, and analyses generally focus on specific treatment settings or regimens. Although it is probable that a large proportion of participants who were lost to follow‐up achieved SVR, it is likely that some ceased or failed treatment, and virological failure remains undetected. Many patients who are lost to follow‐up may eventually receive confirmation of cure testing, although in many cases this may be opportunistic at time of re‐engagement with health services. With the high effectiveness of DAAs, loss to follow‐up is of little concern in those who adhere to treatment. Further work is needed to identify how resources can be directed toward those who have ceased treatment and likely remain viremic. Higher proportions of missing SVR data among people with recent IDU is consistent with findings from previous real‐world studies. A cohort from the Vancouver Infectious Disease Center, consisting of people with a history of IDU (n = 291), found those who injected in the past 6 months were disproportionately represented in loss to follow‐up.( 35 ) Loss to follow‐up in people who inject drugs is of particular concern, as it may mask early cessation of treatment or poor adherence, leading to treatment failure and ongoing injecting behavior that may increase likelihood of reinfection.( 36 ) It may also lead to uncertainty over which treatment to use when the person represents with viremia, as reinfection and failure are often indistinguishable in this context. Reasons for increased loss to follow‐up in this marginalized population are complex, reflecting the need for strategies to overcome barriers such as access to services, stigma, and lack of social and mental health support.( 37 , 38 ) There may also be a small proportion who present for SVR testing but are unable to have blood taken due to difficult venous access.( 39 ) This may be overcome by capillary fingerstick testing through dried blood spot collection or point‐of‐care HCV‐RNA technologies such as GeneXpert. Point‐of‐care testing can provide results within 1 hour( 40 ) and has demonstrated acceptability among people who inject drugs.( 41 , 42 ) The integration of point‐of‐care testing in which people who inject drugs are already engaged, such as needle exchange programs, may enhance confirmation of cure and monitoring for reinfection.
The strongest predictor of loss to follow‐up in REACH‐C was year of treatment initiation, increasing over time. There are several factors likely contributing to this observation. First, although all participants were followed for a minimum of 12 months following treatment, those who initiated early were followed for a longer period of time and therefore had more opportunity to present for testing. Second, there may be some apathy toward SVR testing that has developed over time following observation of consistently high cure. Third, and likely most impactful, the characteristics of the population initiating treatment evolved over time to encompass more vulnerable groups, including those who are younger, indigenous, and with recent IDU. The initial wave of treatment initiations were largely older, stable patients engaged in care who had been “warehoused” awaiting DAAs. This group posed only a small risk to public health and HCV elimination compared to younger people with active IDU. The increasing representation of individuals with recent IDU in REACH‐C is consistent with findings from the Australian Needle and Syringe Program Survey, which reported increasing uptake of HCV treatment in the prior 12 months among people who inject drugs (from 22% in 2016 to 44% in 2019).( 22 ) The high efficacy of DAA therapy among those with recent IDU (93%) and increasing uptake correlates with a more than halving of HCV prevalence (51% in 2015 to 20% in 2018) and early evidence of declining national HCV incidence reported in Australia between 2015 and 2018. 22 , 43 Despite the progress made in this marginalized population, we have demonstrated the challenge with maintaining engagement in follow‐up care. Implementation of additional strategies, such as cultural competency for clinicians working with Indigenous populations, should be prospectively explored to counter loss to follow‐up.
A major strength of REACH‐C is the inclusion of diverse clinical services across all Australian jurisdictions and data collected on consecutive treatment initiations, incorporating a large sample representative of the wider Australian‐treated population. Real‐world observational cohorts have inherent limitations, including challenges with obtaining complete data. Routine documentation varied across centers, and retrospective extraction of characteristics for some participants (e.g., indigenous identification, IDU, and OAT) was not possible. There was no standardized follow‐up after treatment completion, with each center using their own strategies to maintain engagement in care. Although attempts were made to locate HCV‐RNA results collected through services outside the REACH‐C network, it is possible that some participants categorized as loss to follow‐up had subsequent testing at alternate clinical services, the results of which were not obtained. Furthermore, we did not systematically capture treatment adherence or alcohol consumption, which may confound factors associated with SVR. It should also be noted that most general practice treatment within REACH‐C was initiated by high HCV caseload general practitioners who likely differ in their HCV knowledge to the average Australian general practitioner.
In summary, treatment response was high in a diverse population receiving care through a broad range of services following universal access to DAA therapy in Australia. Loss to follow‐up presents a real‐world challenge that may compromise HCV elimination, with younger people who inject drugs more likely to disengage from care following treatment. Evaluation and implementation of innovative strategies are required to retain people at risk of loss to follow‐up in care.
Supporting information
Fig S1
Fig S2
Table S1
Table S2
Acknowledgment
The authors thank current and past researchers and staff for their contribution to research acknowledge the following members of the study group:
Protocol Steering Committee: David Iser (chair; Scope Gastroenterology, Melbourne, Australia), Gail Matthews (University of New South Wales [UNSW] Sydney, Sydney, Australia), Gregory Dore (UNSW Sydney, Sydney, Australia), Josh Hanson (Cairns and Hinterland Hospital and Health Service, Cairns, Australia), James O’Beirne (Sunshine Coast Hospital and Health Service, Sunshine Coast, Australia), Phillip Read (The Kirketon Road Center, Sydney, Australia), Anne Balcomb (Prince Street Medical Center, Orange, Australia), Joanne Carson (UNSW Sydney, Sydney, Australia), Jasmine Yee (UNSW Sydney, Sydney, Australia), and Philippa Marks (UNSW Sydney, Sydney, Australia).
Coordinating Center: Jasmine Yee (study coordinator), Joanne Carson (research assistant), Gail Matthews (coprincipal investigator), Gregory Dore (coprincipal investigator), Behzad Hajarizadeh (senior lecturer), Marianne Byrne (clinical trials manager), and Philippa Marks (clinical trials manager).
Site Principal Investigators: Jeffery Post (The Albion Center, Sydney, Australia), Joseph Doyle (The Alfred Hospital, Melbourne, Australia), Robert Batey (Alice Springs Hospital, Alice Springs, Australia), John Smart (Asquith Medical Center, Sydney, Australia), Olivia Dawson (Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine, Sydney, Australia), Sonja Hill (Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine, Sydney, Australia), Mark Douglas (Blacktown Hospital, Sydney, Australia), Marianne Martinello (Blacktown Hospital, Sydney, Australia), Mark Montebello (Brookvale Community Health Center, Sydney Australia; and Royal North Shore Community Health Center, Sydney, Australia), Patricia Collie (Bulgarr Ngaru Medical Aboriginal Corporation, Grafton, Australia; and Toormina Medical Center, Coffs Harbor, Australia), Richard Hallinan (The Byrne Surgery, Sydney, Australia), Josh Hanson (Cairns and Hinterland Hospital and Health Service, Cairns, Australia), Gail Snelgar (Dubbo Community Health Center, Dubbo, Australia), David Baker (East Sydney Doctors, Sydney, Australia), Sam Galhenage (Fiona Stanley Hospital, Perth, Australia), Tuck Meng Soo (Interchange General Practice, Canberra, Australia), Phillip Read (Kirketon Road Center, Sydney, Australia), Rohan Bopage (The Langton Center, Sydney, Australia), John Faros (The Langton Center, Sydney, Australia), Lucy Cooper (Matthew Talbot Hostel, Sydney, Australia), Anne Balcomb (Prince Street Medical Center, Orange, Australia), Renjy Nelson (The Queen Elizabeth Hospital, Adelaide, Australia), David Shaw (Royal Adelaide Hospital, Adelaide, Australia), Jane Davies (Royal Darwin Hospital, Darwin, Australia), Mark Wilson (Royal Hobart Hospital, Hobart, Australia), David Iser (Scope Gastroenterology, Melbourne, Australia), William Pratt (Shoalhaven District Memorial Hospital, Shoalhaven, Australia), Stephen Hinton (St. John of God Hospital, Bunbury, Australia), Gregory Dore (St Vincent’s Hospital, Sydney, Australia), James O’Beirne (Sunshine Coast University Hospital, Sunshine Coast, Australia), Amanda Wade (University Hospital Geelong, Geelong, Australia), Helen Van Gessel (Western Australia Country Health Service, Albany, Australia), Leonie Davidson (Western Australia Country Health Service, Broome, Australia) Miranda Dibdin (Western Australia Country Health Service, Broome, Australia), and Micaela Lucas (Wollongong Hospital, Wollongong, Australia).
Site Coordinators: Raghib Ahmad (The Albion Center, Sydney, Australia), Denise Smith (The Albion Center, Sydney, Australia), Khim Tan (Alice Springs Hospital, Alice Springs, Australia), Christine Roder (The Alfred Hospital, Melbourne, Australia; and University Hospital Geelong, Geelong, Australia), Brendan Harney (The Alfred Hospital, Melbourne, Australia), Susan Holdaway (Blacktown Hospital, Sydney, Australia), Jayde Walsh (Brookvale Community Health Center, Sydney Australia), Penny Fox (Cairns and Hinterland Hospital and Health Service, Cairns, Australia), Roshanak Mousavi (East Sydney Doctors, Sydney, Australia), Wendy Lam (Fiona Stanley Hospital, Perth, Australia), Rupa Pudasaini Dahal (Fiona Stanley Hospital, Perth, Australia), Philip Habel (Interchange General Practice, Canberra, Australia), Rosie Gilliver (Kirketon Road Center, Sydney, Australia), Edmund Silins (Kirketon Road Centre, Sydney, Australia), Jessica Ackerman (The Langton Center, Sydney, Australia), Rachel Deacon (The Langton Center, Sydney, Australia), Edmund Hall (The Langton Center, Sydney, Australia), Arlene Everson (Matthew Talbot Hostel, Sydney, Australia), Margery Milner (The Queen Elizabeth Hospital, Adelaide, Australia), Catherine Ferguson (Royal Adelaide Hospital, Adelaide, Australia), Jaclyn Tate‐Baker (Royal Darwin Hospital, Darwin, Australia), Jane Bradshaw (Royal Hobart Hospital, Hobart, Australia), Gai Duncan (Royal North Shore Community Health Center, Sydney Australia), Gilbert Baluran (Royal North Shore Community Health Center, Sydney Australia), Belinda Watson (Shoalhaven District Memorial Hospital, Shoalhaven, Australia), Camilla Hey (St. John of God Hospital, Bunbury, Australia), Rebecca Hickey (St. Vincent’s Hospital, Sydney, Australia), Clare Orme (Sunshine Coast University Hospital, Sunshine Coast, Australia), and Silvie Miczkova (Western Australia Country Health Service, Albany, Australia).
Supported by the Australian Government Department of Health and Ageing.
Potential conflict of interest: Iser advises and is on the speakers’ bureau of AbbVie, Gilead Sciences, and MSD. Read is on the speakers’ bureau of and received grants from Gilead Sciences. He is on the speakers’ bureau of AbbVie. Dore received grants from Gilead Sciences and AbbVie. Doyle consults for and received grants from Gilead Sciences and AbbVie. He received grants from Merck. Matthews received grants from Gilead Sciences and AbbVie.
Contributor Information
Gail V. Matthews, Email: gmatthews@kirby.unsw.edu.au.
the REACH‐C Study Group:
David Iser, Gail Matthews, Gregory Dore, Josh Hanson, James O’Beirne, Phillip Read, Anne Balcomb, Joanne Carson, Jasmine Yee, Philippa Marks, Jasmine Yee, Joanne Carson, Gail Matthews, Gregory Dore, Behzad Hajarizadeh, Marianne Byrne, Philippa Marks, Jeffery Post, Joseph Doyle, Robert Batey, John Smart, Olivia Dawson, Sonja Hill, Mark Douglas, Marianne Martinello, Mark Montebello, Patricia Collie, Richard Hallinan, Josh Hanson, Gail Snelgar, David Baker, Sam Galhenage, Tuck Meng Soo, Phillip Read, Rohan Bopage, John Faros, Lucy Cooper, Anne Balcomb, Renjy Nelson, David Shaw, Jane Davies, Mark Wilson, David Iser, William Pratt, Stephen Hinton, Gregory Dore, James O’Beirne, Amanda Wade, Helen Van Gessel, Leonie Davidson, Miranda Dibdin, Micaela Lucas, Raghib Ahmad, Denise Smith, Khim Tan, Christine Roder, Brendan Harney, Susan Holdaway, Jayde Walsh, Penny Fox, Roshanak Mousavi, Wendy Lam, Rupa Pudasaini Dahal, Philip Habel, Rosie Gilliver, Edmund Silins, Jessica Ackerman, Rachel Deacon, Edmund Hall, Arlene Everson, Margery Milner, Catherine Ferguson, Jaclyn Tate‐Baker, Jane Bradshaw, Gai Duncan, Gilbert Baluran, Belinda Watson, Camilla Hey, Rebecca Hickey, Clare Orme, and Silvie Miczkova
References
- 1. The Kirby Institute . Annual Surveillance Report of HIV, viral hepatitis, STIs 2016. Kensington, NSW, Australia: The Kirby Institute; 2016, 63, 65, 122. https://kirby.unsw.edu.au/sites/default/files/kirby/report/SERP_Annual‐Surveillance‐Report‐2016_UPD170627.pdf. Accessed 15 April 2020. [Google Scholar]
- 2. Ward RP, Kugelmas M. Using pegylated interferon and ribavirin to treat patients with chronic hepatitis C. Am Fam Physician 2005;72:655‐662. [PubMed] [Google Scholar]
- 3. Dore GJ, Hajarizadeh B. Elimination of hepatitis C virus in Australia: laying the foundation. Infect Dis Clin North Am 2018;32:269‐279. [DOI] [PubMed] [Google Scholar]
- 4. Australian Government Department of Health . National Hepatitis C Testing Policy. Sydney, Australia; 2020. http://testingportal.ashm.org.au/files/National_Hepatitis_C_Testing_Policy_Oct2020.pdf. Accessed 12 April 2021. [Google Scholar]
- 5. Razali K, Thein HH, Bell J, Cooper‐Stanbury M, Dolan K, Dore G, et al. Modelling the hepatitis C virus epidemic in Australia. Drug Alcohol Depend 2007;91:228‐235. [DOI] [PubMed] [Google Scholar]
- 6. Hajarizadeh B, Grebely J, Matthews GV, Martinello M, Dore GJ. Uptake of direct‐acting antiviral treatment for chronic hepatitis C in Australia. J Viral Hepat 2018;25:640‐648. [DOI] [PubMed] [Google Scholar]
- 7. Dore GJ, Feld JJ. Hepatitis C virus therapeutic development: in pursuit of “perfectovir.” Clin Infect Dis 2015;60:1829‐1836. [DOI] [PubMed] [Google Scholar]
- 8. Falade‐Nwulia O, Suarez‐Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct‐acting agent therapy for hepatitis c virus infection: a systematic review. Ann Intern Med 2017;166:637‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ji F, Yeo YH, Wei MT, Ogawa E, Enomoto M, Lee DH, et al. Sustained virologic response to direct‐acting antiviral therapy in patients with chronic hepatitis C and hepatocellular carcinoma: a systematic review and meta‐analysis. J Hepatol 2019;71:473‐485. [DOI] [PubMed] [Google Scholar]
- 10. Haridy J, Wigg A, Muller K, Ramachandran J, Tilley E, Waddell V, et al. Real‐world outcomes of unrestricted direct‐acting antiviral treatment for hepatitis C in Australia: the South Australian statewide experience. J Viral Hepat 2018;25:1287‐1297. [DOI] [PubMed] [Google Scholar]
- 11. Haridy J, Iyngkaran G, Nicoll A, Muller K, Wilson M, Wigg A, et al. Outcomes of community‐based hepatitis C treatment by general practitioners and nurses in Australia via remote specialist consultation. Intern Med J 2020. Sep 6. 10.1111/imj.15037. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12. Lee A, Hanson J, Fox P, Spice G, Russell D, Boyd P. A decentralised, multidisciplinary model of care facilitates treatment of hepatitis C in regional Australia. J Virus Erad 2018;4:160‐164. [PMC free article] [PubMed] [Google Scholar]
- 13. Bartlett SR, Fox P, Cabatingan H, Jaros A, Gorton C, Lewis R, et al. Demonstration of near‐elimination of hepatitis C virus among a prison population: the Lotus Glen Correctional Centre Hepatitis C Treatment Project. Clin Infect Dis 2018;67:460‐463. [DOI] [PubMed] [Google Scholar]
- 14. Read P, Lothian R, Chronister K, Gilliver R, Kearley J, Dore GJ, et al. Delivering direct acting antiviral therapy for hepatitis C to highly marginalised and current drug injecting populations in a targeted primary health care setting. Int J Drug Policy 2017;47:209‐215. [DOI] [PubMed] [Google Scholar]
- 15. White L, Azzam A, Burrage L, Orme C, Kay B, Higgins S, et al. Facilitating treatment of HCV in primary care in regional Australia: closing the access gap. Frontline Gastroenterol 2019;10:210‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Williams J, Lucarelli N, Nicoll A, Lubel J. Real‐world Australian data reflect very high sustained virologic response at 12 weeks with direct acting antiviral therapy for hepatitis C and suggests highly achievable even in those without an end‐of‐treatment response. Intern Med J 2019;49:666‐669. [DOI] [PubMed] [Google Scholar]
- 17. Tsertsvadze T, Gamkrelidze A, Nasrullah M, Sharvadze L, Morgan J, Shadaker S, et al. Treatment outcomes of patients with chronic hepatitis C receiving sofosbuvir‐based combination therapy within national hepatitis C elimination program in the country of Georgia. BMC Infect Dis 2020;20:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Australian Government Department of Health General Statement for Drugs for the Treatment of Hepatitis C. Pharm Benefits Scheme. 2017. https://www.pbs.gov.au/healthpro/explanatory‐notes/general‐statement‐pdf/General‐Statement‐for‐Drugs‐for‐the‐Treatment‐of‐Hepatitis‐C.pdf. Accessed 15 April 2020.
- 19. The Kirby Institute . Monitoring hepatitis C treatment uptake in Australia. Sydney, UNSW, Australia: The Kirby Institute; 2019:1‐11. https://kirby.unsw.edu.au/sites/default/files/kirby/report/Monitoring‐hep‐C‐treatment‐uptake‐in‐Australia_Iss10‐JUN19.pdf. Accessed 23 March 2020. [Google Scholar]
- 20. Razavi H, Sanchez Gonzalez Y, Yuen C, Cornberg M. Global timing of hepatitis C virus elimination in high‐income countries. Liver Int 2020;40:522‐529. [DOI] [PubMed] [Google Scholar]
- 21. Martinello M, Yee J, Bartlett SR, Read P, Baker D, Post JJ, et al. Moving towards hepatitis C microelimination among people living with human immunodeficiency virus in Australia: the CEASE Study. Clin Infect Dis 2020;71:1502‐1510. [DOI] [PubMed] [Google Scholar]
- 22. Heard S, Iverson J, Geddes L, Maher L. Australian needle and syringe program survey 25 year national data report 1995‐2019. Sydney, Australia; 2020. https://kirby.unsw.edu.au/sites/default/files/kirby/report/ANSPS_25‐Year‐National‐Data‐Report‐1995‐2019.pdf. Accessed 10 February 2021. [Google Scholar]
- 23. Australian Government Department of Health . Fifth National Hepatitis C Strategy 2018‐2022. https://www1.health.gov.au/internet/main/publishing.nsf/Content/ohp‐bbvs‐1/$File/Hep‐C‐Fifth‐Nat‐Strategy‐2018‐22.pdf. Accessed 15 April 2020.
- 24. The Kirby Institute . REACH‐C Online Specialist Consultation. 2016. https://reach‐c.ashm.org.au/about‐the‐project/. Accessed 17 April 2020.
- 25. Pourmarzi D, Smirnov A, Hall L, Thompson H, FitzGerald G, Rahman T. Enablers and barriers for the provision of community‐based HCV treatment: a case study of a real‐world practice. J Viral Hepat 2020;27:484‐496. [DOI] [PubMed] [Google Scholar]
- 26. Marshall AD, Grebely J, Dore GJ, Treloar C. Barriers and facilitators to engaging in hepatitis C management and DAA therapy among general practitioners and drug and alcohol specialists—the practitioner experience. Drug Alcohol Depend 2020;206:107705. [DOI] [PubMed] [Google Scholar]
- 27. Mangia A, Milligan S, Khalili M, Fagiuoli S, Shafran SD, Carrat F, et al. Global real‐world evidence of sofosbuvir/velpatasvir as simple, effective HCV treatment: analysis of 5552 patients from 12 cohorts. Liver Int 2020;40:1841‐1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Welzel TM, Nelson DR, Morelli G, Di Bisceglie A, Reddy RK, Kuo A, et al. Effectiveness and safety of sofosbuvir plus ribavirin for the treatment of HCV genotype 2 infection: results of the real‐world, clinical practice HCV‐TARGET study. Gut 2017;66:1844‐1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sulkowski MS, Vargas HE, Di Bisceglie AM, Kuo A, Reddy KR, Lim JK, et al. Effectiveness of simeprevir plus sofosbuvir, with or without ribavirin, in real‐world patients with HCV genotype 1 infection. Gastroenterology 2016;150:419‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yek C, de la Flor C, Marshall J, Zoellner C, Thompson G, Quirk L, et al. Effectiveness of direct‐acting antiviral therapy for hepatitis C in difficult‐to‐treat patients in a safety‐net health system: a retrospective cohort study. BMC Med 2017;15:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cachay ER, Mena A, Morano L, Benitez L, Maida I, Ballard C, et al. Predictors of hepatitis C treatment failure after using direct‐acting antivirals in people living with human immunodeficiency virus. Open Forum Infect Dis 2019;6:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Darvishian M, Wong S, Binka M, Yu A, Ramji A, Yoshida EM, et al. Loss to follow‐up: a significant barrier in the treatment cascade with direct‐acting therapies. J Viral Hepat 2020;27:243‐260. [DOI] [PubMed] [Google Scholar]
- 33. Bao Y, Larney S, Peacock A, Colledge S, Grebely J, Hickman M, et al. Prevalence of HIV, HCV and HBV infection and sociodemographic characteristics of people who inject drugs in China: a systematic review and meta‐analysis. Int J Drug Policy 2019;70:87‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Heal 2017;5:e1192‐e1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alimohammadi A, Holeksa J, Thiam A, Truong D, Conway B. Real‐world efficacy of direct‐acting antiviral therapy for HCV infection affecting people who inject drugs delivered in a multidisciplinary setting. Open Forum Infect Dis 2018;5:ofy120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. Risk of late relapse or reinfection with hepatitis C virus after achieving a sustained virological response: a systematic review and meta‐analysis. Clin Infect Dis 2015;62:683‐694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bajis S, Dore GJ, Hajarizadeh B, Cunningham EB, Maher L, Grebely J. Interventions to enhance testing, linkage to care and treatment uptake for hepatitis C virus infection among people who inject drugs: a systematic review. Int J Drug Policy 2017;47:34‐46. [DOI] [PubMed] [Google Scholar]
- 38. Ingiliz P, Wehmeyer MH, Boesecke C, Schulze Zur Wiesch J, Schewe K, Lutz T, et al. Reinfection with the hepatitis C virus in men who have sex with men after successful treatment with direct‐acting antivirals in Germany: current incidence rates, compared with rates during the interferon era. Clin Infect Dis 2020;71:1248‐1254. [DOI] [PubMed] [Google Scholar]
- 39. Day CA, White B, Thein HH, Doab A, Dore GJ, Bates A, et al. Experience of hepatitis C testing among injecting drug users in Sydney, Australia. AIDS Care Psychol Socio‐Medical Asp AIDS/HIV 2008;20:116‐123. [DOI] [PubMed] [Google Scholar]
- 40. Lamoury FMJ, Bajis S, Hajarizadeh B, Marshall AD, Martinello M, Ivanova E, et al. Evaluation of the Xpert HCV viral load finger‐stick point‐of‐care assay. J Infect Dis 2018;217:1889‐1896. [DOI] [PubMed] [Google Scholar]
- 41. Bajis S, Maher L, Treloar C, Hajarizadeh B, Lamoury FMJ, Mowat Y, et al. Acceptability and preferences of point‐of‐care finger‐stick whole‐blood and venepuncture hepatitis C virus testing among people who inject drugs in Australia. Int J Drug Policy 2018;61:23‐30. [DOI] [PubMed] [Google Scholar]
- 42. Williams B, Howell J, Doyle J, Thompson AJ, Draper B, Layton C, et al. Point‐of‐care hepatitis C testing from needle and syringe programs: an Australian feasibility study. Int J Drug Policy 2019;72:91‐98. [DOI] [PubMed] [Google Scholar]
- 43. The Kirby Institute . National update on HIV, viral hepatitis and sexually transmissible infections in Australia: 2009–2018. 2020. .https://kirby.unsw.edu.au/sites/default/files/kirby/report/National‐update‐on‐HIV‐viral‐hepatitis‐and‐STIs‐2009‐2018.pdf. Accessed 12 April 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1
Table S2


