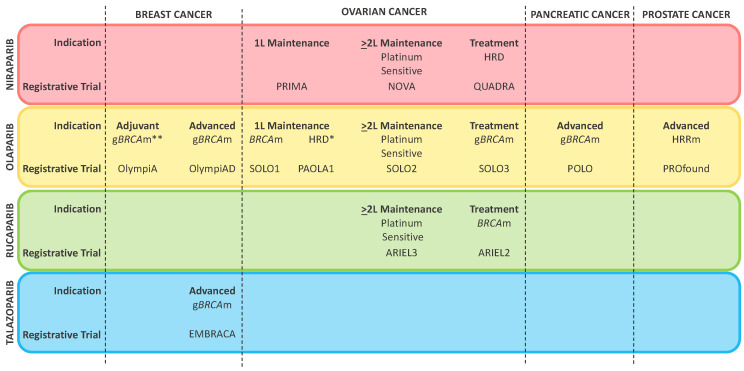
Figure 1.
Currently approved indications of PARP inhibitors according to the Food and Drug Administration. * Olaparib in combination with bevacizumab; ** This indication has not been approved yet, but has been granted an accelerated review process by the Food and Drug Administration on 30 November 2021. Figure Legend: 1L first line therapy; 2L second line therapy; BRCAm: BRCA mutated (germline or somatic); gBRCAm: germline BRCA mutated; HRD homologous recombination deficiency; HRRm: homologous recombination repair mutated.