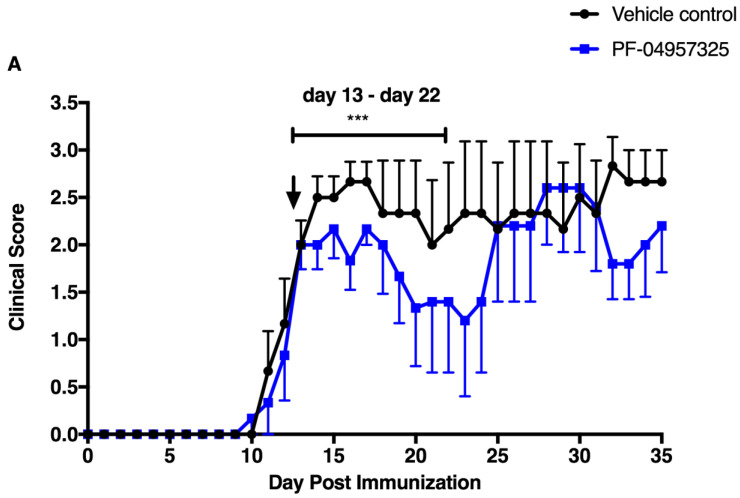
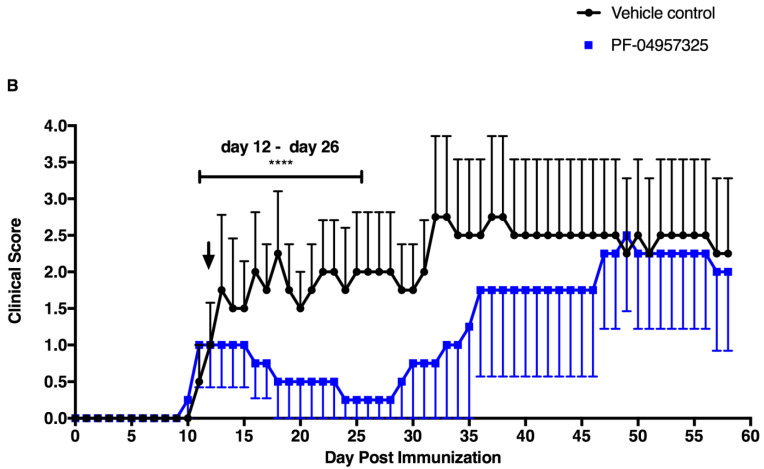
Figure 1.
Treatment with the PDE8 inhibitor PF-04957325 treats clinical signs of EAE in a therapeutic manner. (A) C57BL/6 mice with an EAE score of at least 1 were treated with the vehicle control in an equivalent volume of DMSO and PBS to the corresponding inhibitor dose (vehicle control), or PF-04957325 (10 mg/kg/dose diluted in DMSO and PBS) subcutaneously 3 times per day, from day 13 to day 22, as indicated by the bar in the figure. The arrow indicates the day on which the treatments were initiated. The data represent the mean clinical scores (mean ± SEM) for each day (n = 6 mice per group) *** p = 0.0007. (B) C57BL/6 EAE mice were implanted with Alzet mini-osmotic pumps filled with either the vehicle alone (50% DMSO and 50% PBS) or PF-04957325 (with a continuous release rate of 15.5 mg/kg/day) in a vehicle on day 12 post-immunization. The treatment duration based on the physical properties of the pumps was 14 days, as indicated by the bar in the figure. The arrow indicates the day on which the treatments were initiated. The data represent the mean clinical scores (mean ± SEM) for each day (n = four mice per group) **** p < 0.0001.