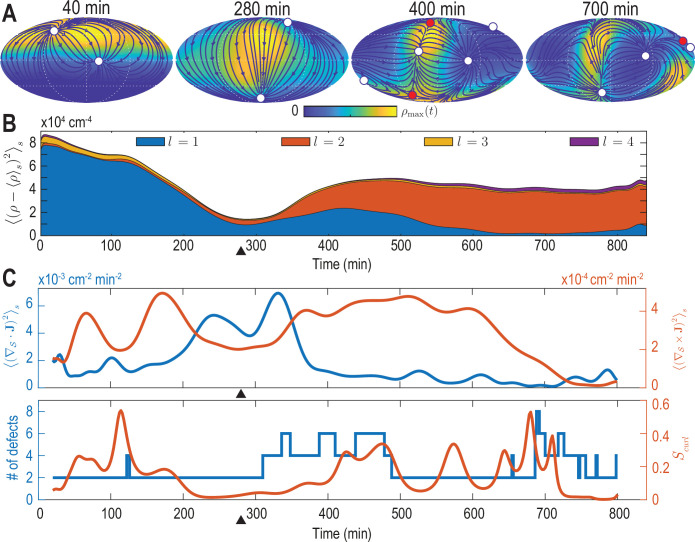
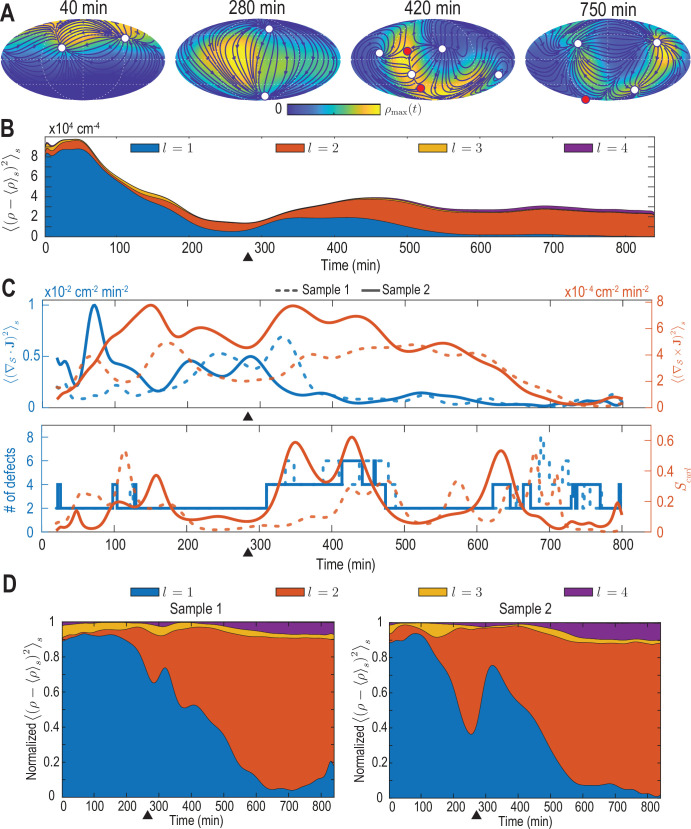
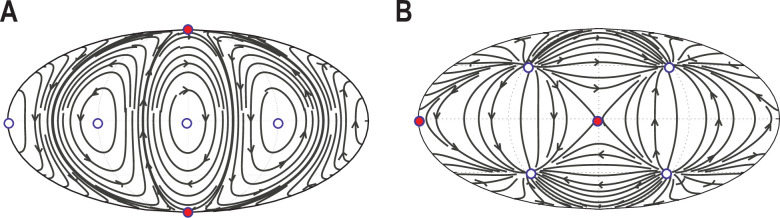
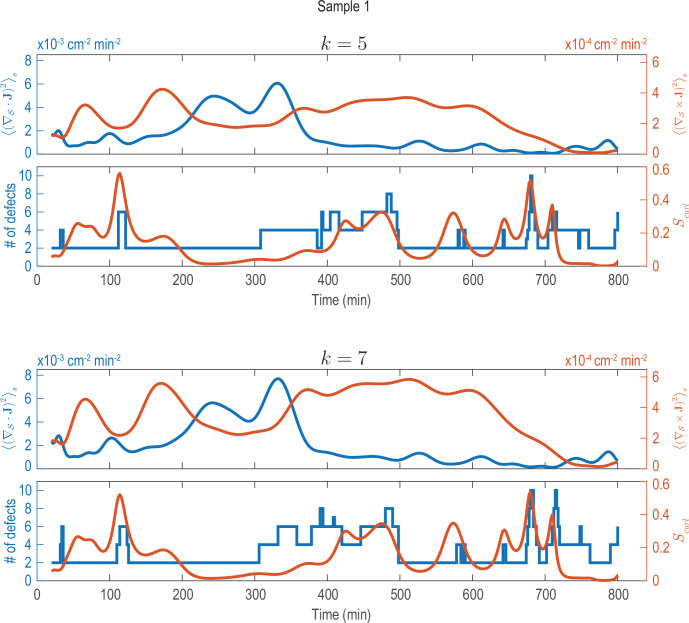
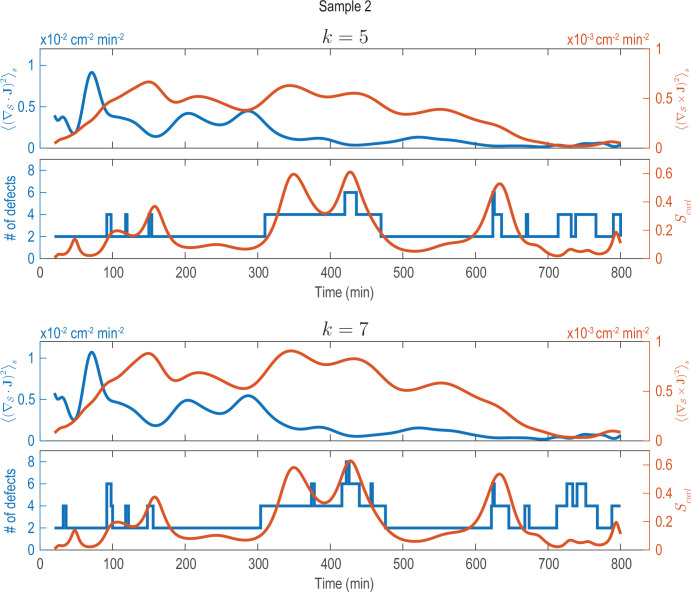
Figure 2. Mode signatures of developmental symmetry breaking and topological defects in cellular flux.
(A) Two-dimensional Mollweide projection of the compressed coarse-grained density field (colormap) and of the coarse-grained cell flux (streamlines) at different time points of zebrafish gastrulation.White circles depict topological defects of charge +1 in the flux vector field, red circles depict defects with charge -1. The total defect charge is 2 at all times. Defects are seen to ‘lead’ the large-scale motion of cells and later localize mostly along the curve defined by the forming spine. Animal pole (AP) and ventral pole (VP) are located at top and bottom, respectively. (B) Density fluctuations as a function of developmental time (see Equation 9), broken down in contributions from different harmonic modes . The underlying symmetry breaking is highlighted prominently by this representation: During the first 75% of epiboly (0–280 min) cells migrate away from, but are still mostly located near the animal pole, presenting a density pattern with polar symmetry (). During the following convergent extension phase cells converge towards a confined elongated region that is ‘wrapped’ around the yolk, corresponding to a density pattern with nematic symmetry (). Black triangles indicate transition from epiboly to convergent extension. (C) Comparison of surface averaged divergence and curl of the cellular flux computed via Equation 10a and Equation 10b, respectively (top). A relative curl amplitude computed from these quantities via Equation 11 correlates with the appearance of an increased number of topological defects in the cell flux (bottom), suggesting that incompressible, rotational cell flux is associated with the formation of defects.