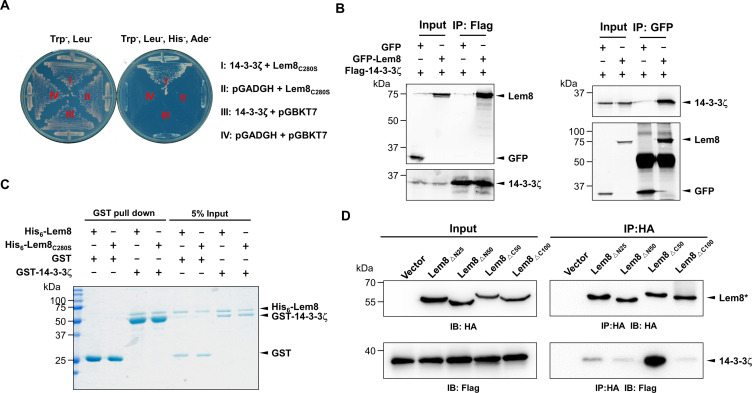
Figure 2. The interactions between Lem8 and 14-3-3ζ.
(A) Interactions between Lem8 and 14-3-3ζ detected by yeast two-hybrid assay. Yeast strains harboring the indicated constructs were streaked on Leu− and Trp− medium to select for plasmids (left) or on Leu−, Trp−, Ade−, and His− medium to assess the interactions (right). Images were acquired after 3-day incubation at 30°C. (B) Lem8 and 14-3-3ζ form a protein complex in mammalian cells. Total lysates of HEK293T cells transfected with the indicated plasmid combinations were immunoprecipitated with a Flag-specific antibody (left panels) or GFP-specific antibodies (right panels), and the precipitates were probed with both Flag and GFP antibodies. Similar results were obtained from at least three independent experiments and the data shown here were from one representative experiment. (C) Lem8 directly interacts with 14-3-3ζ. GST-14-3-3ζ was incubated with His6-Lem8 or His6-Lem8C280S, and the potential protein complex was captured by glutathione beads for 1 hr at 4°C. After extensive washing, bound proteins were solubilized with sodium dodecyl sulfate (SDS) loading buffer, and proteins were detected by Coomassie brilliant blue staining after being resolved by SDS/polyacrylamide gel electrophoresis PAGE. Similar results were obtained from at least three independent experiments and the data shown here were from one representative experiment. (D) Interactions between 14-3-3ζ and Lem8 deletion mutants. Lysates of 293T cells expressing Flag-14-3-3ζ and each of the HA-tagged deletion Lem8 were subjected to immunoprecipitation with the anti-HA antibody and the presence of 14-3-3ζ in the precipitates was probed with the Flag-specific antibody. Similar results were obtained from at least three independent experiments and the data shown here were from one representative experiment.