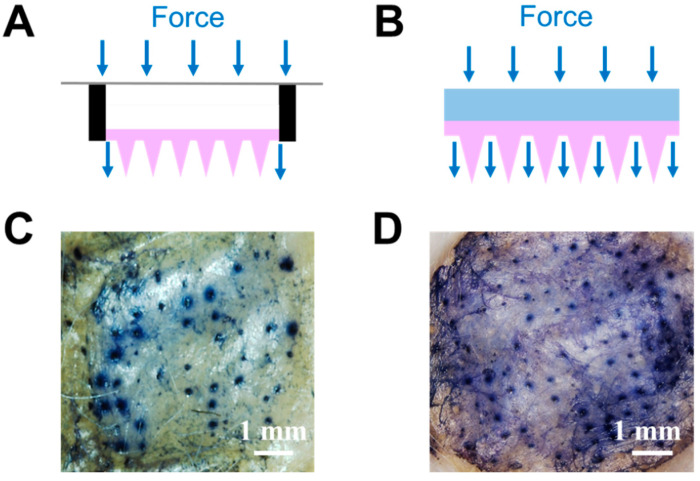
Figure 6.
(A) Schematic of microneedles with a hollow drug reservoir, and (B) “cake-like” type 3 hybrid MNs with porous gel serving as a reservoir. Mouse skin was treated with (C) microneedles with a hollow drug reservoir or (D) “cake-like structure” type 3 hybrid MNs with porous gel reservoir for 10 min and stained with trypan blue to indicate the in vitro skin insertion ability.