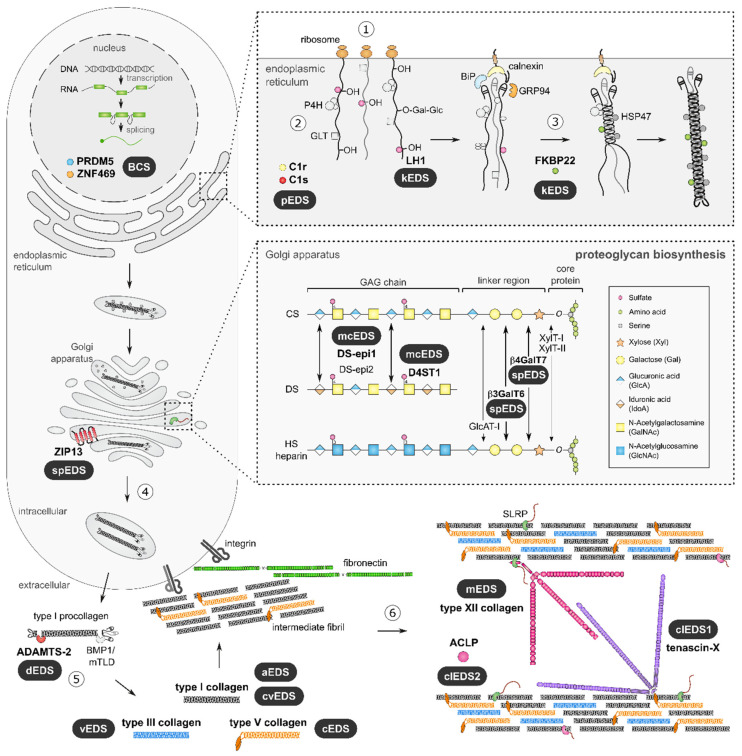
Figure 2.
Collagen and proteoglycan biosynthesis in the context of EDS. Defective molecules associated with EDS are indicated in bold, and the respective EDS type is indicated. Fibrillar collagen biosynthesis is initiated by transcription and translation of pro-α-chains (step 1). Nascent pro-α-chains are co- and post-translationally modified by several modifying enzymes in the endoplasmic reticulum (ER), such as proline and lysine hydroxylases and galactosyltransferases (step 2). Triple helix formation starts by the association of the C-terminal propeptides of three pro-α-chains and propagates towards the N-terminus in a zipperlike fashion during which several molecular chaperones assist (step 3). Trimeric procollagen molecules aggregate laterally, are transported in secretory vesicles and are secreted into the extracellular environment (step 4). Collagen molecules are formed by removal of the N- and C-propeptides by ADAMTS-2 and BMP-1/mTLD, respectively (step 5). These collagens subsequently assemble into highly ordered striated fibrils. The assembly of collagen fibrils is tissue-specific and requires several assisting proteins (step 6). Fibronectin and integrins serve as organizers of fibril assembly at the plasma membrane. At the cell surface, some collagens, including type V collagen, function as nucleators and initiate immature fibril assembly. Type V collagen co-assembles with type I collagen to form heterotypic fibrils with the entire triple helical domain of type V collagen embedded within the fibril. The partially processed N-propeptide domain of type V collagen protrudes to the fibril surface where it controls fibrillogenesis by sterically hindering the addition of collagen monomers. Intermediate fibrils are deposited into the ECM and stabilized by interactions with regulators, such as the small leucine-rich proteoglycan (SLRP) decorin, tenascin-X and type XIII collagen. These molecules influence the rate of assembly, size and structure of the collagen fibrils. Subsequent fibril growth occurs through linear and lateral fusion of intermediate collagen fibrils, which are stabilized by intra- and inter-molecular crosslinks. Proteoglycan biosynthesis is initiated by the synthesis of a core protein, which is then modified by several Golgi-resident enzymes. First, a common linker region in formed by the addition of four monosaccharides. Formation of this tetrasaccharide linker region begins with the stepwise addition of a xylose (Xyl) residue to a serine residue of the core protein, catalyzed by xylosyltransferase-I and -II (XylT-I/-II). Subsequently, two galactose (Gal) residues are added by galactosyltransferase-I (GalT-I or β4GalT7) and galactosyltransferase-II (GalT-II or β3GalT6). Finally, the addition of a glucuronic acid (GlcA), catalyzed by glucuronosyltransferase-I (GlcAT-I) completes the formation of the linker region. The alternating addition of either N-acetylglucosamine (GlcNAc) or N-acetylgalactosamine (GalNAc) and GlcA to the nascent GAG-chain result in the formation of heparan sulfate (HS) proteoglycans and chondroitin sulfate (CS)/dermatan sulfate (DS) proteoglycans. The GAG-chains are further modified by epimerization and sulfation reactions. The epimerization of GlcA towards iduronic acid (IdoA), which is catalyzed by DS epimerases-1 and -2 (DS-epi1 and DS-epi2) is necessary for the formation of DS. Subsequently, dermatan 4-O-sulfotransferase 1 (D4ST1) is able to catalyze 4-O-sulfation of GalNAc, which prevents back-epimerization of the adjacent IdoA.