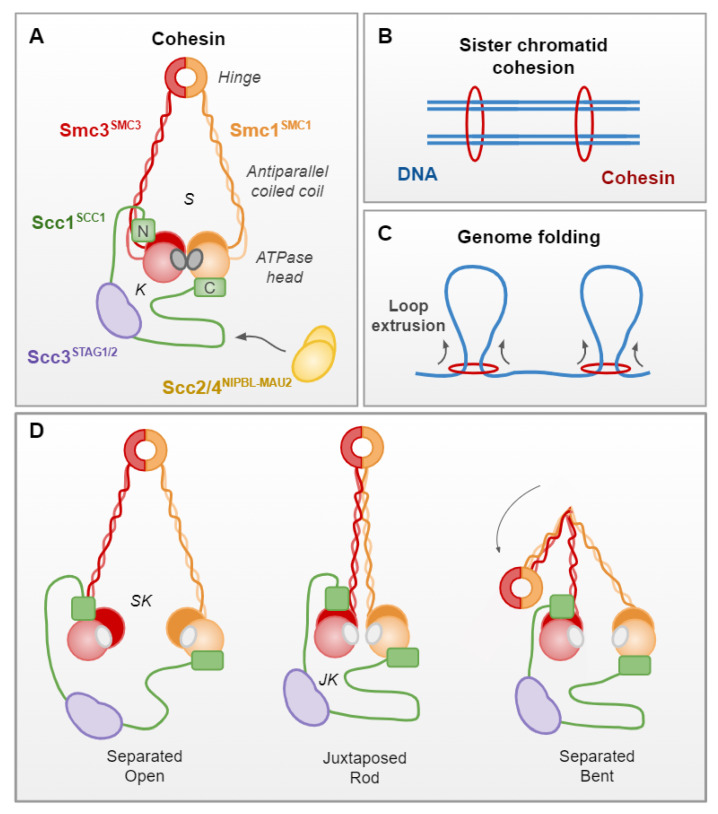
Figure 2.
Cohesin structure and molecular functions. (A) The cohesin complex, shown in the ATP-bound state, has four core subunits: the structural maintenance of chromosomes proteins, Smc1 and Smc3, the kleisin Scc1 and the kleisin-associating Scc3STAG1/2. The loading complex Scc2/4NIPBLA/NIPBLB–Mau2 interacts with cohesin through Scc1. SMC proteins consist of ATPase head and hinge domains, and a long antiparallel coiled-coil arm. In the ATP-bound state, closed SMC (S) and kleisin (K) compartments are observed. (B) Cohesin holds sister chromatids together from S phase to anaphase. (C) Cohesin forms long-range intrachromatid loops, likely by a symmetrical extrusion process. (D) Cohesin can exist in multiple conformations determined by ATP binding (SMC heads engaged) and hydrolysis (SMC heads juxtaposed/separated). When separated, the coiled-coil arms generate one open SMC–kleisin (SK) compartment. In the juxtaposed state, the SMC coiled coils align, generating a rod-shaped complex, with a juxtaposed kleisin (JK) compartment. Alignment of the coiled coil is permissive to bending at an elbow region within the arms, bringing the hinge domain into close contact with the SMC3 head domain.