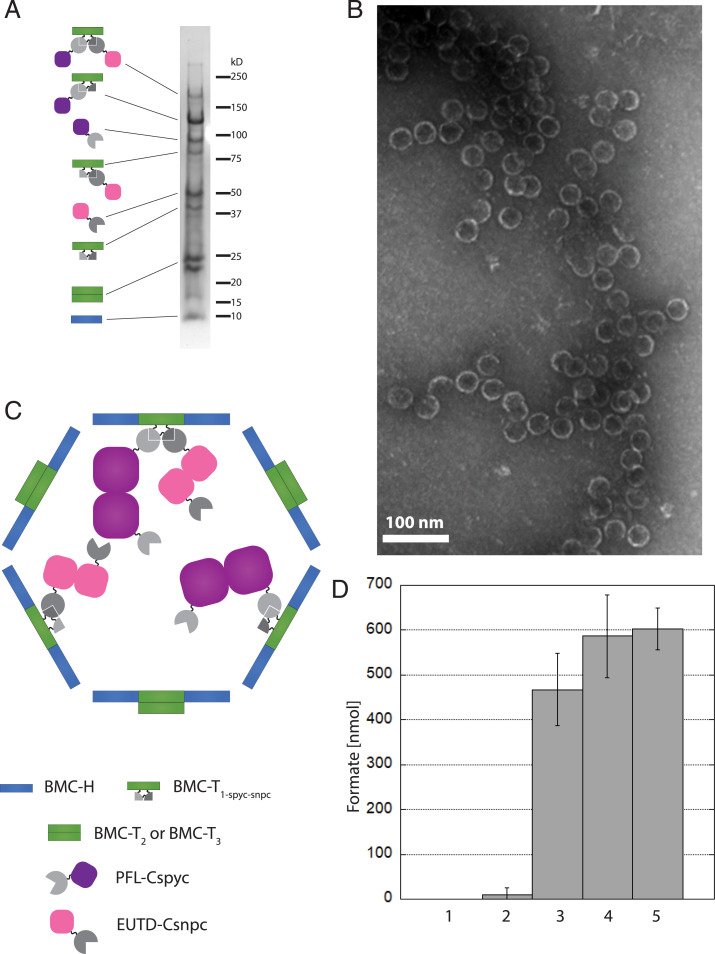
Fig. 4.
Purification of complete assembled active sFUTs. (A) Coomassie-stained SDS/PAGE gel. T1-spyt-snpt-6xHis in conjugation with PFL-Cspyc and EutD-Csnpc (∼170 kDa), T1-spyt-snpt-6xHis conjugated to PFL-Cspyc (∼120 kDa), PFL-Cspyc (∼95 kDa), EutD-Csnpc conjugated to T1-spyt-snpt-6xHis (∼80 kDa), EutD-Csnpc (∼50 kDa), T1-spyt-snpt-6xHis (∼40 kDa), T2 and T3 (22 kDa and 23 kDa, respectively) as well as the BMC-H (10 kDa). Proteins have been identified by mass spectrometry. (B) TEM images of the sFUT compartments. Structures observed at ∼40 nm in diameter. (C) Model of a complete assembled sFUT wiffleball. PFL and EutD form dimers. Not all T1-spyt-snpt-6xHis conjugation points are occupied, probably due to steric hindrance. (D) Activity of sFUT wiffleballs. Lane 1: no sFUT control; lane 2: inactivated sFUT by exposure to oxygen after isolation; lane 3: sFUT; lane 4: sFUT + CoA; lane 5 sFUT + acetyl-CoA. Formation of formate is shown per sFUT wiffleball over a 15-min time frame when provided with 1 µmol pyruvate. Addition of 1 mM CoA boosts activity, as well as addition of 1 mM acetyl-CoA. Acetyl-CoA can only be used by the PFL if EutD converts it to acetyl phosphate and CoA, thus indicating EutD activity.