Figure 1.
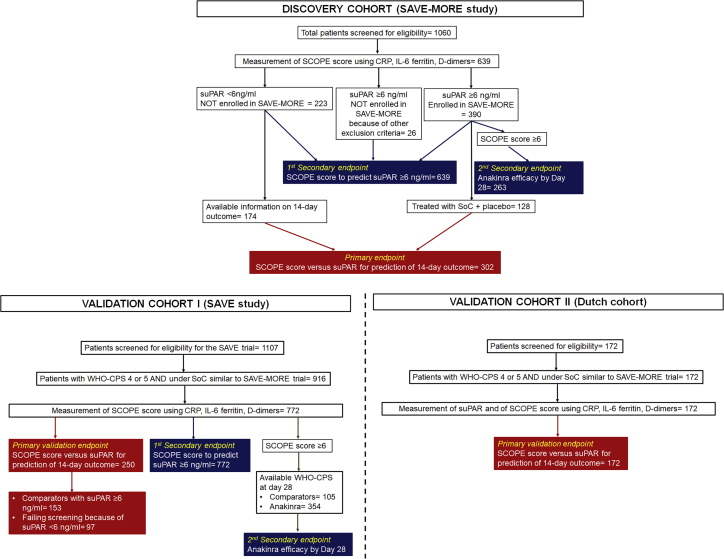
Flowchart of the discovery and the validation cohorts
The discovery cohort was recruited from patients screened for eligibility for participation in the SAVE-MORE trial and for which the screening samples were available for the measurement of the SCOPE score. The samples of patients with suPAR of less than 6 ng/mL and of patients with suPAR of 6 ng/mL or more treated in the SAVE-MORE trial with placebo were analyzed for the primary endpoint, i.e., prediction of progression into severe respiratory failure or death within the first 14 days (302 total). Samples from all 639 patients were analyzed for the ability of the SCOPE score to predict suPAR levels; and 263 patients with SCOPE score of 6 or more were analyzed for the efficacy of anakinra treatment. The validation cohort I came from the SAVE trial and it was analyzed for the same endpoints as the discovery cohort. The validation cohort II came from the Netherlands and it was analyzed for the primary endpoint. SCOPE, Severe COvid Prediction Estimate; SoC, standard of care; suPAR, soluble urokinase plasminogen activator receptor; WHO-CPS, World Health Organization Clinical Progression Scale.