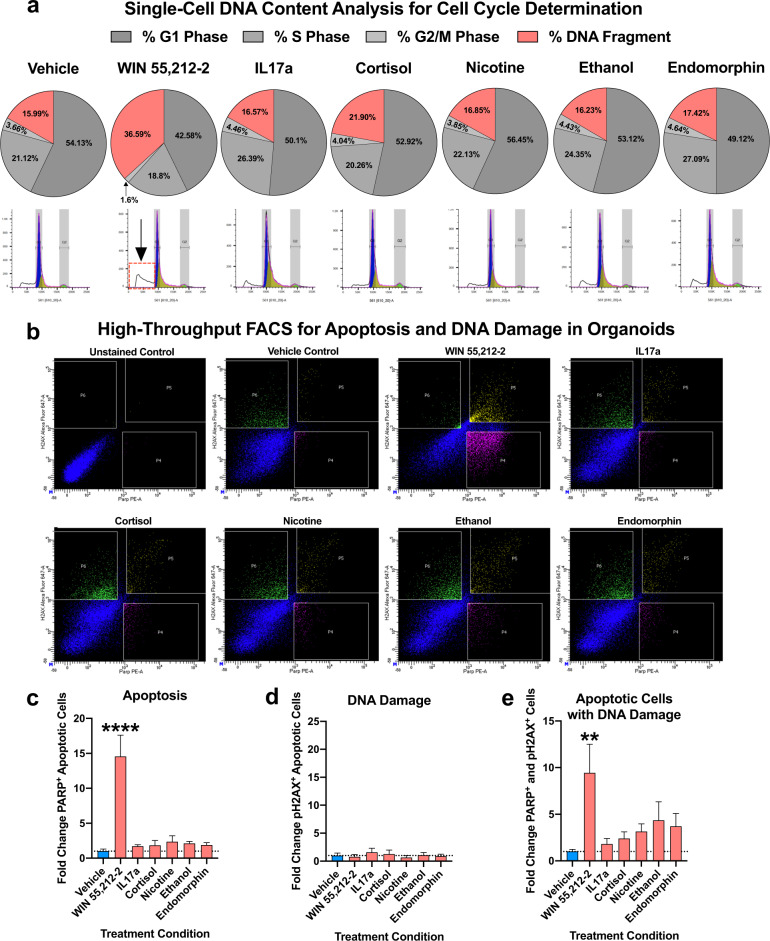
Fig. 4. Analysis if DNA content, DNA damage, and apoptosis induction within enviromimetic human-derived forebrain organoids.
a Increased DNA fragmentation in organoids treated with WIN 55,212-2. To examine if our narcotic and neuropsychiatric-related treatments altered the cell cycle of proliferating cells within human-derived dorsal forebrain organoids, we adapted a single-cell DNA content analysis. Briefly, whole organoids were dissociated to a single-cell suspension and labeled with the fluorescent DNA-intercalating agent Propidium Iodide (PI). DNA content below our G1 peak serves as a proxy of cell death due to DNA fragmentation/digestion by endonucleases [93]. No major alterations in the proportion of proliferating cells or DNA fragmentation were detected in our IL17a, cortisol, nicotine, ethanol, and endomorphin treatment groups. However, a robust effect of WIN 55,212-2 treatment was detected for the DNA content, whereby WIN 55,212-2 selectively increased DNA fragmentation in our human-derived dorsal forebrain organoids. This indicated that, of our various narcotic and neuropsychiatric-related enviromimetic treatments, the cannabinoid agonist WIN 55,212-2 may selectively induce neurotoxicity and thus cell death within developing human-derived dorsal forebrain organoids. DNA histograms are provided for all treatment groups underneath their respective pie-charts, which delineate the proportion of cells in G1 phase, S phase, G2/M phase, and those which exhibit DNA fragmentation (i.e., dead and/or dying cells). For reference, a red-box and arrow identifies the DNA fragmentation peak in dorsal forebrain organoids treated with WIN 55,212-2. b–e, Validation of death and DNA damage in organoids treated with WIN 55,212-2. To provide orthogonal validation of our DNA content analysis, we adapted a multi-panel FACS assay to provide a higher-resolution analysis of cell death and DNA damage. Following a series of fixation, permeabilization, and re-fixation steps (designed to gain access to the nucleus), cells were labeled with an Alexa647-conjugated phosphorylated H2AX antibody to detect the presence of DNA damage machinery and a PE-conjugated cleaved PARP antibody for detection of cell death induction. As shown in bar graphs, the endocannabinoid receptor agonist WIN 55,212-2 was the only treatment to significantly increase cell death (PARP-PE + cells, or gate P4 in flow charts) within human-derived dorsal forebrain organoids. There was no increase in P2HAX + healthy cells (i.e., gate P6 in flow charts) that exhibited no evidence of apoptosis amongst any of our treatment groups. However, there was a significant increase in the proportion of DNA damaged apoptotic cells (H2AX + and PARP + double-positive cells, depicted by gate P5 in flow charts) selectively within human-derived dorsal forebrain organoids treated with WIN 55,212-2. For all panels, n = 4–6 iPSC donors x n = 7 treatment groups for a total n of = 34–41 experimental samples for flow cytometry analysis across experiments. All gates/quadrants in flow charts were pseudorandomly labeled. **** denotes p < 0.00001 and ** denotes p < 0.01. Bar graphs represent mean ± SEM.