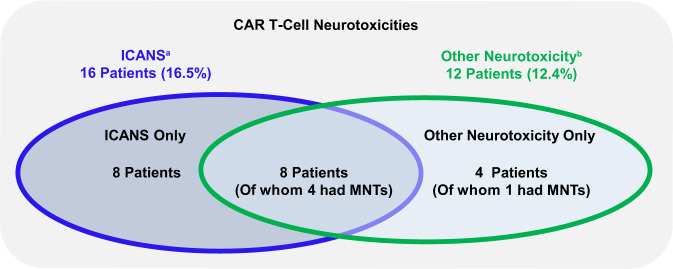
Fig. 1. Overview of CAR T-cell neurotoxicities in CARTITUDE-1.
aICANS per ASTCT consensus criteria. bNeurotoxicity as assessed by the investigator to be related to cilta-cel occurring after a period of recovery from CRS and/or ICANS. ICANS and other neurotoxicity events are not mutually exclusive; eight (8.2%) patients experienced both ICANS and other neurotoxicity events of any grade. Patients in the other-neurotoxicity group (n = 12) include the subset with MNTs (n = 5). ASTCT American Society for Transplantation and Cellular Therapy, CAR chimeric antigen receptor, CRS cytokine release syndrome, ICANS immune effector cell-associated neurotoxicity syndrome, MNTs movement and neurocognitive treatment-emergent adverse events.