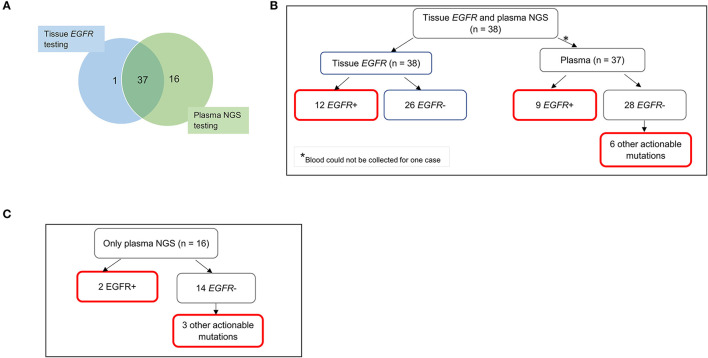
Figure 2.
Diagnostic yield from molecular testing of tissue and plasma samples for 54 patients with NSCLC. (A) Nearly all 38 patients with informative tissue EGFR testing underwent plasma NGS testing (except one). An additional 16 patients had only plasma NGS testing done, due to inadequate tissue biopsy samples for molecular testing or non-informative results from tissue testing. (B) Findings of EGFR sensitizing mutations and other actionable mutations in cases with both tissue EGFR and plasma NGS results. Six other actionable mutations from plasma NGS testing included MET exon 14 skipping (n = 1), BRAF p.V600E (n = 1), BRAF p.K601E (n = 1), KRAS p.G12D (n = 2), and EGFR exon 20 insertion (n = 1). (C) Clinically actionable findings in cases with only plasma NGS testing. Boxes outlined in red indicate clinically actionable diagnostic yield from all testing modalities. EGFR, epidermal growth factor receptor; NGS, next-generation sequencing.