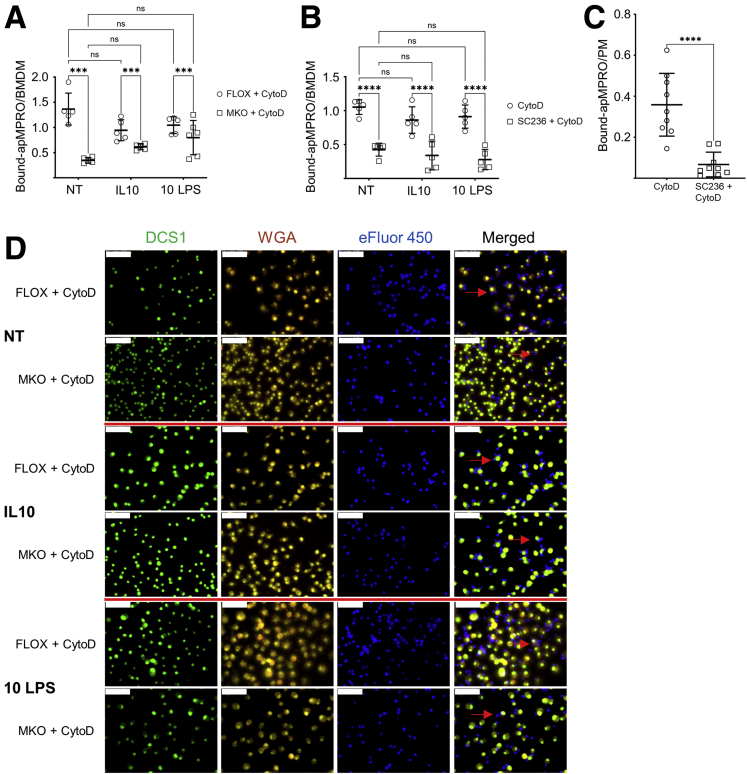
Figure 5.
COX2 modulates efferocytosis by BMDMs and PMs in part by affecting the binding capacity for apoptotic neutrophils. For all panels, cytochalasin D (CytoD) was added 30 minutes prior to the addition of apMPRO neutrophils, to prevent the internalization of cell surface bound apoptotic cells. (A) FLOX and Cox2 MKO BMDMs were either left unpolarized (NT) or polarized for 48 hours with IL-10 or low-dose LPS (10 LPS). Binding of apMPRO cells to the surface of CytoD-treated FLOX and Cox2 MKO macrophages was determined as bound apMPRO per BMDM. (B) BMDMs isolated from BL6 mice were treated and assessed as described for panel A, with the exception that COX2 was chronically inhibited with SC236. (C) Thioglycolate-elicited PMs from BL6 mice were treated with vehicle or SC236 for 7 days, and the PM binding capacity for apMPRO was determined as in panel A. (D) Representative data from the experiment presented in panel A. BMDMs were stained with nuclear green DCS1 as well as with the cell membrane dye WGA Texas Red-X conjugate; MPRO cells were stained with Cell Proliferation Dye eFluor 450. ×40 magnification. Red arrows point to apMPRO cells bound to the macrophage cell membrane, but not internalized. Scale bar = 50 um. For panels A–C, 5–9 fields were analyzed from 3 biological replicates per condition. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. (A, B) Two-way analysis of variance with Tukey’s multiple comparisons test and adjusted P values. (C) Student’s unpaired t test.