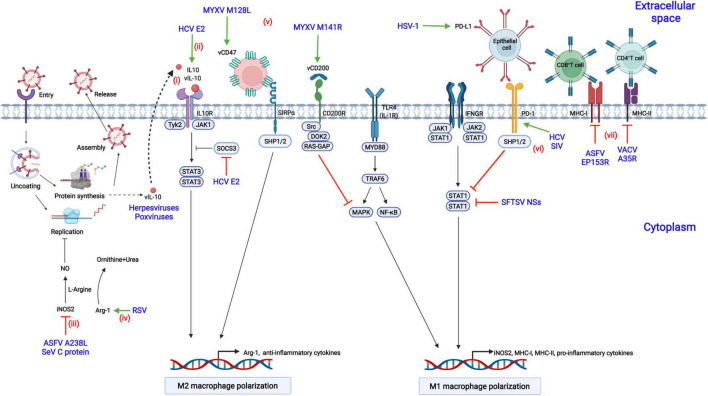
FIGURE 3.
Modulation of macrophage polarization by viral proteins. (i) Herpesviruses or poxviruses encode the homolog of IL-10 (vIL-10), which binds to IL-10R, triggers the activation of STAT3, and contributes to M2 macrophage polarization; (ii) Hepatitis C virus (HCV) encodes the E2 protein, which upregulates IL-10 production by increasing the phosphorylation of STAT3 and reducing the expression of SOCS3; (iii) African swine fever virus (ASFV) infection results in the suppression of nitrogen oxide (NO) production by downregulating inducible nitric oxide synthase 2 (iNOS2) via the A238L protein; (iv) Respiratory syncytial virus (RSV) inhibits NO production by decreasing the expression of arginase 1 (Arg-1) and the level of L-arginine; (v) Myxoma virus (MYXV) encodes the M141R (the viral CD200 homolog) and M128L (the viral CD47 homolog) proteins, which suppress the M1 phenotype polarization of macrophages; (vi) Acute infection with herpes simplex virus type 1 (HSV-1) and chronic infection with HCV or simian immunodeficiency virus (SIV) lead to the increased expression of PD-L1 or PD-1, inhibiting STAT1 activation and M1 macrophage polarization; (vii) The vaccinia virus (VACV) A35R protein suppresses the antiviral responses of T cells by negatively regulating the major histocompatibility complex class II (MHC-II) antigen presentation, while the ASFV EP153R and the HIV-1 Nef proteins downregulate MHC-I expression.