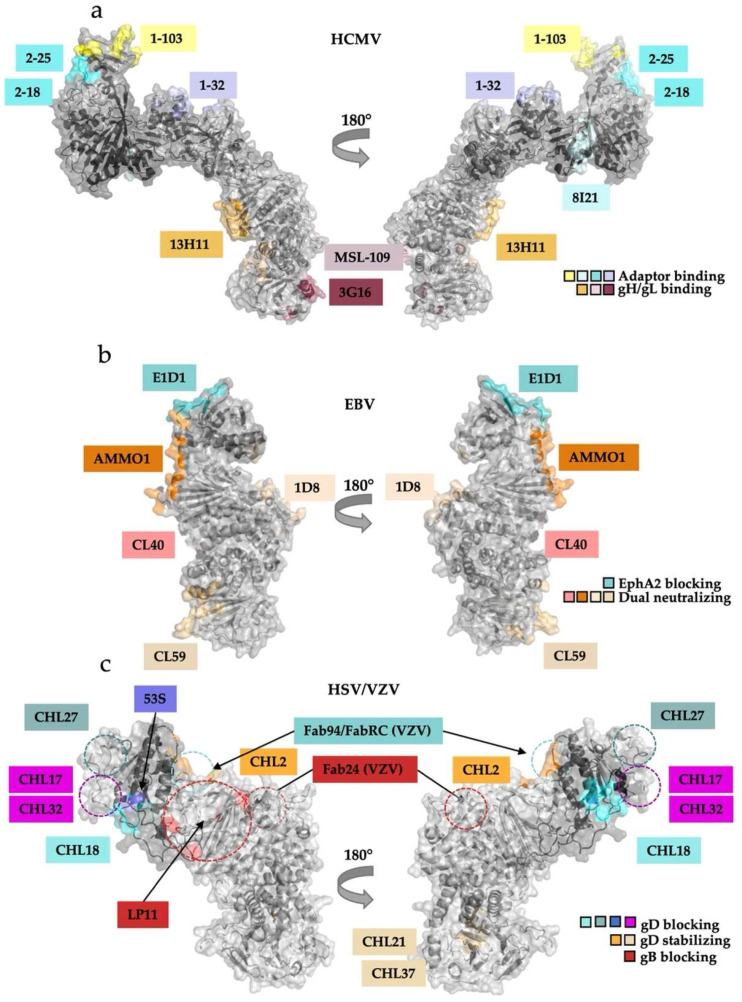
Figure 5.
Antibody binding locations on the gH/gL suggest their mechanisms of action. (a) Antibodies against HCMV pentamer bind either the gH/gL heterodimer or the adaptor subcomplex. 13H11, MSL-109, and 3G16 neutralize infection of both fibroblasts and epithelial cells whereas the pentamer-binding neutralizing Abs only prevent infection of epithelial or endothelial cells. (b) EBV-neutralizing Abs interfere with EphA2 binding but not gp42 or HLA-II binding. E1D1 prevents infection of epithelial cells by sterically interfering with EphA2 engagement by gL. AMMO1, CL40, and CL59 block infection of both epithelial and B-cells without interrupting receptor engagement. (c) A battery of antibodies and antibody fragments (Fabs) that bind gH/gL exert different effects on HSV entry. They can be grouped according to their effects: blocking gD binding, stabilizing gD binding, or blocking gB binding. The effects are localized to specific regions of gH/gL. The existing structure of HSV-2 gH/gL is missing the first 29 amino acids of gH after the signal sequence; the approximate locations of the epitopes that map to this region (CHL27, CHL17, CHL32) are indicated schematically with dashed circles. Approximate locations of the LP11 epitope and the epitopes of three Fabs that bind VZV gH/gL are indicated schematically on the HSV-2 gH/gL structure with dashed circles.