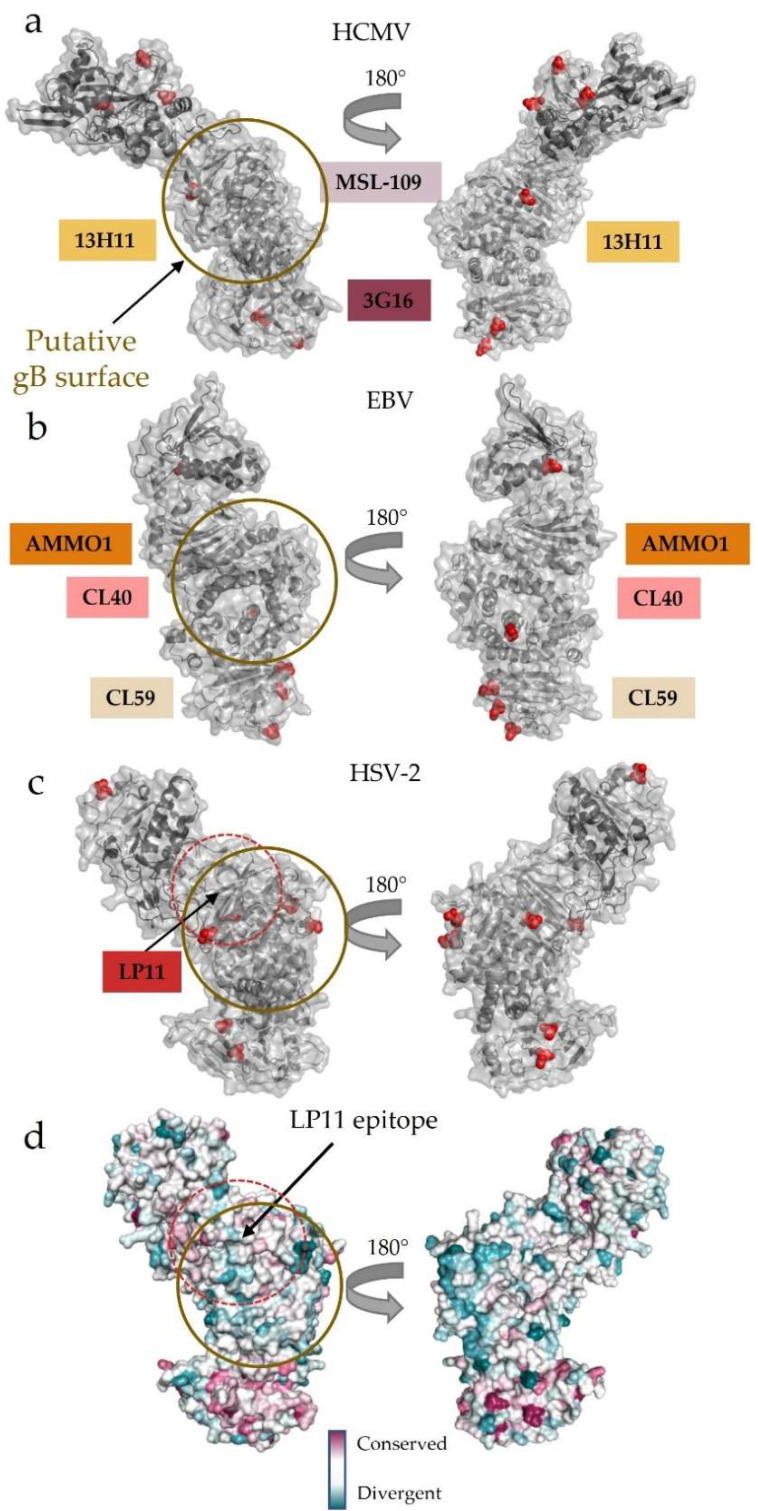
Figure 6.
Surface conservation and glycosylation patterns pinpoint the putative gB-binding region. (a–c) Glycosylation patterns across (a) HCMV, (b) EBV, and (c) HSV-2 gH/gL. The putative gB-binding face, as postulated initially in HSV-2, is remarkably free of glycosylation across the human herpesvirus gH/gL structures currently known. The binding locations of neutralizing antibodies that do not affect host cell receptor binding are indicated along the edge of the heterodimer. These antibodies bind DII, DIII, and DIV in HCMV and EBV gH/gL, while LP11 binds HSV-2 gH/gL at the “kink” between H1 and H2. (d) Surface conservation across herpesviruses mapped onto the HSV-2 gH/gL structure. The N terminus, which is used to engage a wide variety of host-cell receptors and receptor adaptors, is more divergent than the C terminus. The wide face of gH/gL, where LP11–an antibody that disrupts gB-gH/gL interactions–binds is more conserved than the opposite face of the complex. All human herpesviruses require gH/gL and gB interactions to trigger fusion.