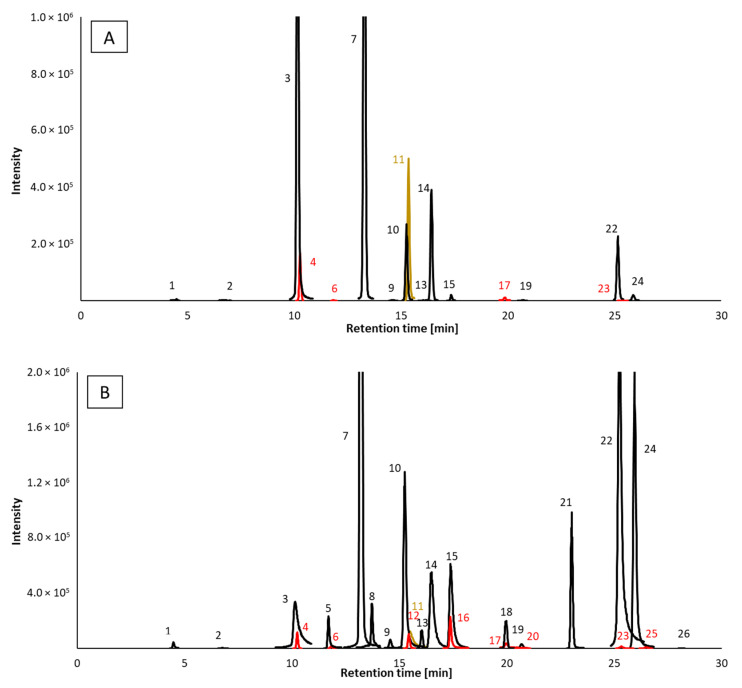
Figure 3.
HPLC-MS/MS separations of phenolic compounds ((A)—sample No. 14; (B)—standard mixture) 1—Pyrogallol; 2—Eugenol; 3—Protocatechuic aldehyde; 4—Tyrosol; 5—Catechin; 6—Homovanillyl alcohol; 7—4-Hydroxybenzaldehyde; 8—Epicatechin; 9—4-Methylcatechol; 10—Vanillin; 11—Ethyl gallate; 12—Coniferyl alcohol; 13—4-Hydroxy-3-methoxyphenylacetone; 14—Syringaldehyde; 15—Scopoletin; 16—Rutin; 17—4-Hydroxy-3-methoxycinnamaldehyde; 18—Tryptophol; 19—2,6-Dimethoxyphenol; 20—Salicylaldehyde; 21—Resveratrol; 22—Ethyl-3,4-dihydroxycinnamate; 23—4-Vinylphenol; 24—Ethyl vanillate; 25—2-Methoxy-4-vinylphenol; 26—4-Ethylguaiacol. Concentration of standards 1mg/L each; chromatograms with different colors correspond to the compounds that coelute but are distinguished by different MRM transitions, as shown in Table S5.