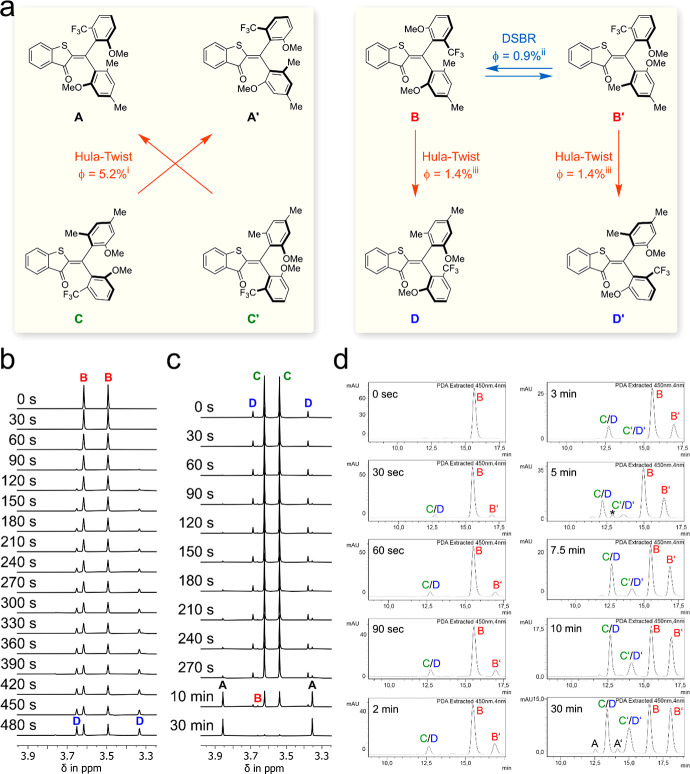
Figure 4.
Photoreactions of HTI 1 during 450 nm irradiation. (a) Photoreactions of 1 and associated quantum yields measured in MeCN solution. iQuantum yield determined taking into account the thermal equilibrium of C and D at 20 °C. iiDSBR = double single-bond rotation, quantum yield indirectly determined by multiplying the ratio of the photoproducts determined by chiral HPLC analysis with the quantum yield for the Hula-Twist reaction from rac-B to rac-D measured at −40 °C. iiiQuantum yield determined at −40 °C. (b) Photoconversion of isomer rac-B in MeCN-d3 solution at −40 °C followed by 1H NMR-spectroscopy (400 MHz). Starting with pure rac-B (top spectrum) only population of isomer rac-D is observed (top to bottom spectra recorded after the indicated irradiation times). (c) Photoconversion of isomer mixture rac-C/rac-D in MeCN-d3 solution at 22 °C followed by 1H NMR-spectroscopy (400 MHz). Starting with a mixture of rac-C/rac-D (top spectrum) almost exclusive population of isomer rac-A is observed (top to bottom spectra recorded after the indicated irradiation times). (d) Photoconversion of isomers B in MeCN solution at −40 °C followed by chiral HPLC. Starting with enantiomerically pure B (chromatogram 1) HPLC runs were conducted after different times of continuous irradiation at −40 °C monitoring the photoisomerization process (chromatograms 2–10). * denotes an impurity.