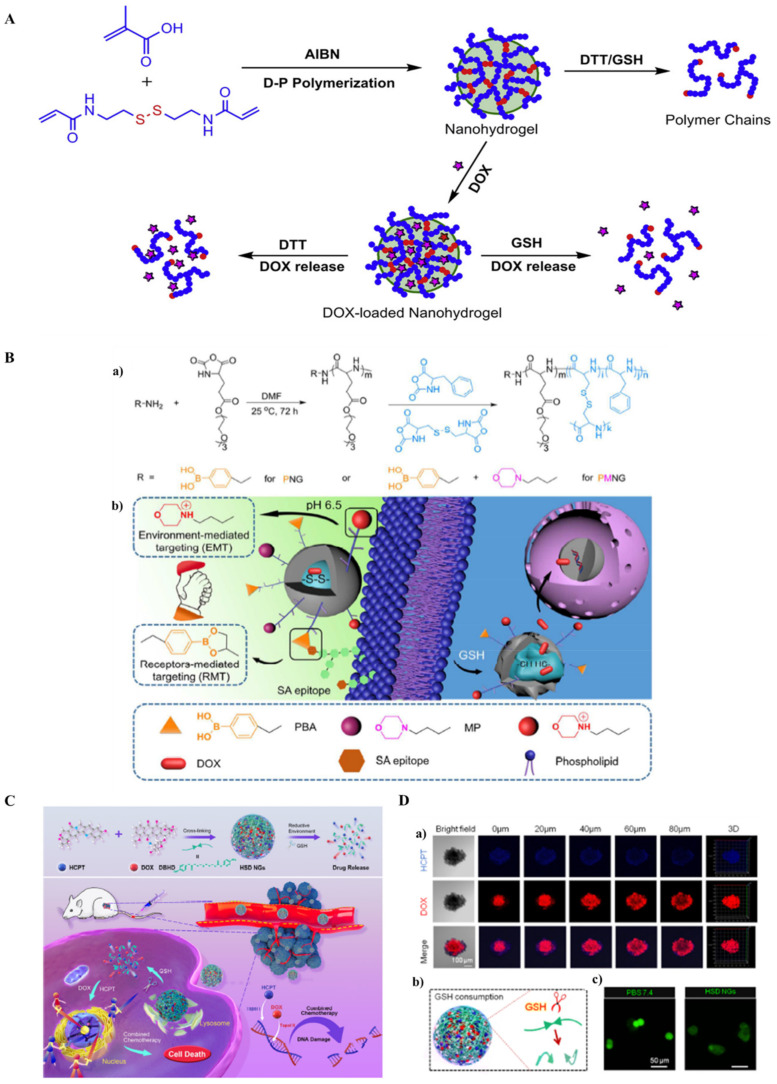
Figure 5.
(A) Illustration of the preparation, biodegradable behavior, and redox stimuli-responsive drug release of the PMAA nanohydrogels. (Reproduced with permission from ref. [120] Copyright Elsevier 2012) (B) Schematic representation of (a) Synthesis route of nanohydrogels. (b) Targeting mechanism of phenylboronic acid and morpholine nanohydrogels. (Reprinted with permission from ref. [121] Copyright @American chemical society 2017) (C) Schematic Illustration of reduction-responsive chemo-capsule-based prodrug nanohydrogel for enhancing the treatment of chemotherapy (D) Deep tumor tissue penetration and GSH response of HSD nanohydrogels. (a) Confocal images of MCSs at different depths that were treated with HSD nanohydrogels for 6 h. (b) Illustration of the disulfide bond breakage in response to GSH. (c) Fluorescence images and fluorescence quantitative results of GSH consumption of 4T1 cells after being treated with HSD nanohydrogels for 6 h. (Reproduced with the permission from ref. [122] Copyright @American chemical society 2021).