Figure 3.
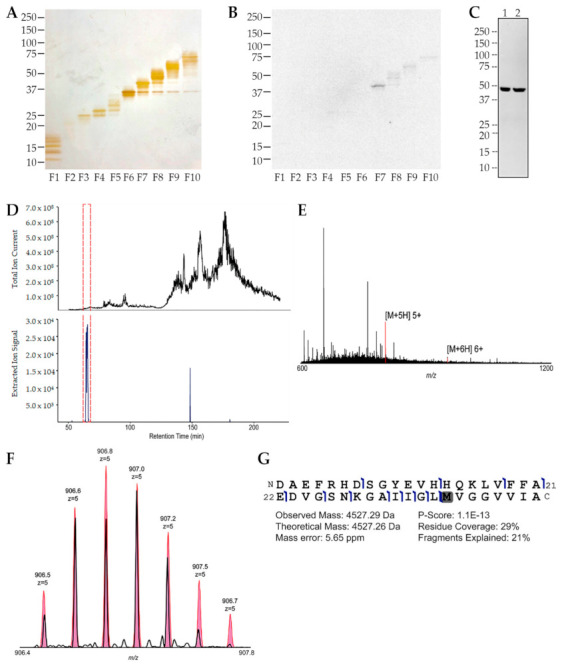
Endogenous AβO expression in the embryonic retina is confirmed by mass spectrometry. (A) Soluble extracts of E16 retina were separated into 10 fractions by GELFrEE and each fraction was analyzed by SDS-PAGE and silver staining to establish protein separation. (B) Western blot of the GELFrEE fractions showed a prominent signal in fraction 7 at approximately 45 kDa detected by the oligomer-selective NU2 antibody. (C) Western blots of unfractionated retina extracts (two separate extracts, 1 and 2, as labeled above each lane) also showed the 45 kDa species. (D) Fraction 7 was analyzed by mass spectrometry, and the panel shows the total-ion-signal chromatogram (top) and a background-subtracted extracted-ion chromatogram (bottom). The panel indicates the only signal with the expected m/z of [M+5H]5+ Aβ peptide. The red dashed box indicates the retention time window in which the oxidized Aβ proteoform was eluted. (E) The full intact mass spectrum is shown at the retention time for Aβ elution. Matched peaks for [M+5H]5+ and [M+4H]4+ Aβ are in red. (F) The select-ion scan (narrow-window) of the [M+5H]5+ Aβ peptide ion (black) is overlaid on its expected isotopic distribution (pink). (G) The fragmentation map is shown for the endogenous Aβ. Fragment ions (blue flags) were matched within 10 ppm mass error to give a specific position where backbone bonds were cleaved. Error in intact mass measurement, the percent of expected fragments observed (coverage) and of fragment peaks matched (explained), and a P-score calculated for confidence in protein identification are indicated. Data confirm that embryonic retina contains an SDS-stable Aβ 10-mer, a result observed in at least four separate experiments.
