Abstract
As an integral component of cardiac tissue, macrophages are critical for cardiac development, adult heart homeostasis, as well as cardiac healing. One fundamental function of macrophages involves the clearance of dying cells or debris, a process termed efferocytosis. Current literature primarily pays attention to the impact of efferocytosis on apoptotic cells. However, emerging evidence suggests that necrotic cells and their released cellular debris can also be removed by cardiac macrophages through efferocytosis. Importantly, recent studies have demonstrated that macrophage efferocytosis plays an essential role in cardiac pathophysiology and repair. Therefore, understanding macrophage efferocytosis would provide valuable insights on cardiac health, and may offer new therapeutic strategies for the treatment of patients with heart failure. In this review, we first summarize the molecular signals that are associated with macrophage efferocytosis of apoptotic and necrotic cells, and then discuss how the linkage of efferocytosis to the resolution of inflammation affects cardiac function and recovery under normal and diseased conditions. Lastly, we highlight new discoveries related to the effects of macrophage efferocytosis on cardiac injury and repair.
Keywords: Cardiac repair, efferocytosis, heart failure, macrophages, myocardial infarction, phagocytosis, resolution of inflammation
INTRODUCTION
In the human body, it is estimated that more than 150 billion cells undergo apoptosis every day which have to be cleared by phagocytes, a process known as programmed cell removal, or “efferocytosis” (1, 2). Efferocytosis is derived from Greek meaning “carry the corpse to the grave,” and initially referred to as phagocytosis of apoptotic cells (3–5). The process of efferocytosis is evolutionarily conserved and occurs for various physiological or pathological reasons, including removing excess cells generated during development and only allowing selected subset of cells to mature and progress; replacing aged cells that come to the end of the lifespan; and eliminating damaged cells during tissue remodeling after injury, which facilitates the progress of tissue repair (6–9). In most tissues, removal of apoptotic/necrotic cells is mediated by either professional or non-professional phagocytes (5). Macrophages are the most common professional phagocytes that are capable of rapidly ingesting and processing multiple corpses in succession (10, 11). Immature dendritic cells are also professional phagocytes, although the capacity of phagocytosis and their access to apoptotic/necrotic corpses are tissue-specific (10, 11). In contrast, non-professional phagocytes generally represent multiple cell types that are “neighbors” to apoptotic/necrotic cells, such as epithelial cells, mesenchymal cells, endothelial cells, and fibroblasts (10, 12). Interestingly, there are specialized phagocytes that exist in specific tissue context, for example, the retinal pigment epithelial cells that remove damaged photoreceptor outer segments, and Sertoli cells that engulf apoptotic germ cells (7).
In adult human and mouse hearts, predominant phagocytes are macrophages that account for ~7% of the nonmyocyte population under healthy condition, and they may represent an even larger fraction in developing hearts (13, 14). The traditional view held that cardiac macrophages were originated from circulating monocytes, which arose from bone marrow-derived hematopoietic stem cells and spleens (15, 16). However, recent studies using fate-mapping and lineage-tracing techniques have revealed that tissue-resident macrophages are established during embryonic development, and they are capable of maintaining the population through self-renewal (17–19). These discoveries have prompted us to better appreciate the diversity of cardiac phagocytes, and sparked significant interests in understanding macrophage efferocytosis in the heart under healthy and disease conditions. In this review, we will summarize the molecular mechanisms that govern the efferocytosis of apoptotic and necrotic cells, and further highlight recent findings on macrophage efferocytosis in cardiac diseases and repair.
MOLECULAR SIGNALING INVOLVED IN EFFEROCYTOSIS OF APOPTOTIC AND NECROTIC CELLS
Despite immense number of cell turnover daily, very few apoptotic cells remain at homeostasis, indicating the presence of an efferocytosis machinery with very high efficiency and capacity. For instance, approximately 7,000 red blood cells are cleared every second by macrophages in mouse spleen and liver (20). Therefore, to ensure such high efficiency and no off-target clearance of healthy cells, efferocytosis has to be tightly regulated and orchestrated through several signaling programs, including: “find-me” signaling, a set of molecules that mediate the recruitment of phagocytes to dying/dead cells; “eat-me” signaling, receptor-mediated uptake of apoptotic and necrotic cells; and digestion signaling, postengulfment processing of cellular material, generally via phagolysosomal degradation. These three stages of signaling cascades are well organized and importantly, disturbance in one or more of these pathways will result in defective efferocytosis and subsequent immune dysfunction and tissue damage (7, 21, 22).
While apoptosis has been considered the dominant mechanism for homeostatic cell turnover, other types of cell death (i.e., gene-regulated necrosis) are also present in various tissues in response to stress conditions (23). Interestingly, recent studies have demonstrated that necrotic cells could be removed by efferocytosis (23). Nonetheless, the signaling pathways for efferocytosis of apoptotic cells and necrotic cells are not entirely the same, as described below.
“Find-me” signaling
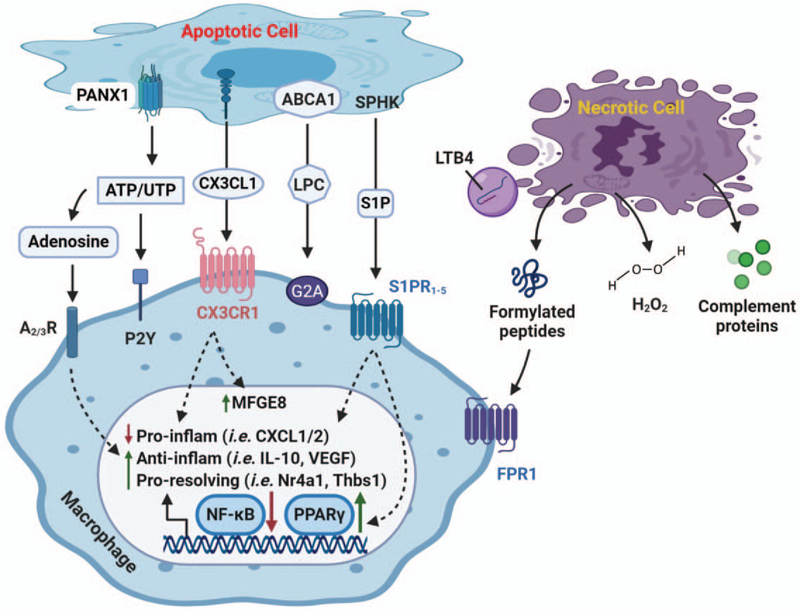
Many factors (i.e., the types of dying/dead cells and phagocytes, stimuli that drive cell death, and the stage of cell death) determine the relevance of individual “find-me” signals in efferocytosis (24). To date, several key “find-me” signaling molecules have been identified including: triphosphate nucleotides (ATP, UTP); chemokines; and lipids such as lysophos-phatidycholine (LPC) and sphingosine-1-phosphate (S1P) (Fig. 1). These “find-me” signaling molecules can be directly released as soluble mediators or exposed on the membrane of apoptotic cells that detach and diffuse in the extracellular environment (23). For example, nucleotides ATP and UTP are exported through the plasma membrane channel pannexin 1 (25). Extracellular ATP may stimulate phagocyte chemotaxis through interaction and upregulation of P2Y purinergic receptors (26, 27). In addition, breakdown of ATP can generate adenosine that affects macrophage recruitment through A3 receptor (28). Interestingly, adenosine has been shown to not only suppress the production of pro-inflammatory cytokines during efferocytosis through adenosine A2 receptors (29, 30), but also upregulate anti-inflammatory mediators (i.e., IL-10, VEGF) and pro-resolving factors (i.e., Nr4a1, Thbs1), leading to the switch of macrophages from pro-inflammatory to antiinflammatory phenotype (31).
Fig. 1. “Find-me” signals and their respective receptors for apoptotic and necrotic cells.
As cells undergo apoptosis, various “find-me” signals [i.e., nucleotides (ATP/UTP), chemokine (CX3CL1), and lipids (LPC and S1P)] are secreted or exposed on the outer leaflet of plasma membrane. Pannexin 1 (PANX1) is important membrane channel responsible for ATP/UTP export, whereas LPC is released by activated ABCA1. Upon binding to receptors, these find-me signals stimulate chemotaxis of macrophages to injured site. Meanwhile, they could promote resolution of inflammation by suppressing gene expression of proinflammatory factors (i.e., CXCL1 and CXCL2), and upregulating anti-inflammatory genes (i.e., IL-10 and VEGF) as well as pro-resolving mediators (i.e., Nr4a1, Thbs1). The efferocytic macrophage-mediated resolution of inflammation is primarily through the inhibition of NF-κB pathway and the activation of PPARγ signaling cascade. Importantly, interaction of CX3CL1 to its receptor also promotes MFGE8, a key bridging molecule of “eat-me” signal. On the other hand, necrotic cells elicit unique “find-me” signaling. For example, formylated peptides are released from damaged mitochondria and interact with FRP1 on macrophages to increase chemotaxis. LTB4 is secreted through MVBs-exosomes that can act in conjunction with formylated peptides. H2O2 is shown to activate Src family of kinase Lyn, leading to enhanced monocyte/macrophage recruitment. In addition, H2O2 can regulate other “find-me” molecules such as formylated peptide, LTB4, and IL-8. Complement proteins can be deposited to target cells or released into extracellular area to interact with neighboring cells. FPR1 indicates formylated peptide receptor 1; LPC, lysophosphatidylcholine; LTB4, leukotriene B4; MVBs, multivesicular bodies; S1P, sphingosine-1-phosphate.
As for CX3C motif chemokine ligand 1 (CX3CL1, also known as fractalkine), it was first identified as “find-me” signal by Truman et al. (32). During apoptosis, CX3CL1 is cleaved by Caspase-3 and released to extracellular environment as recruitment factor for phagocyte chemotaxis through interacting with CX3CR1 (32, 33). In addition, CX3CL1 has been shown to enhance efferocytosis by upregulating the “eat-me” ligand MFGE8 in microgial cells and peritoneal macrophages (34, 35). Recently, Morganti et al. (36) further showed that the soluble form of CX3CL1 could suppress pro-inflammatory cytokine release; whereas its membrane-bound form promoted inflammation. It is important to mention here that Fas-induced chemokines and cytokines (i.e., CCL2/MCP1, IL-8) can also function as “find-me” signal to promote recruitment of phagocytes toward apoptotic cells (37). With regards to lipids (i.e., LPC and S1P), they are apoptosis-specific signals to attract phagocytes. The generation of LPC in apoptotic cells is initiated by Caspase-3 through activating the calcium-independent phospholipase A2 (iPLA2, or PLA2G6) that can in turn metabolize phosphatidylcholine into LPC and subsequently be excreted out via ABCA1-mediated process (38, 39). Interestingly, ABCA1, along with ABCG1 and HDL, can ameliorate oxidative stress and thus protect macrophages against apoptosis during efferocytosis (40). Recent studies have further implicated that LPC could suppress inflammation, evidenced by knockout of the LPC receptor, G2A, causes increased tissue inflammation and systemic autoimmunity (41, 42). Likewise, S1P acts as a potent chemoattractant that is induced by caspase-dependent sphingosine kinase 1 and 2 during apoptosis (43, 44). SIP enhances macrophage recruitment through binding to S1P family of G-protein-coupled receptors (SIPR1–5) (43). Remarkably, S1P could elicit both anti-apoptotic and antiinflammatory effects in macrophages, evidenced by suppression of TNF-α and IL-12 but upregulation of IL-8, IL-10, and VEGF (43, 45). In consistency, recent studies have shown that S1P can promote macrophages toward anti-inflammatory phenotype through the activation of PPARγ and inhibition of NF-κB pathway (43, 46).
In a similar way to apoptotic cells, necrotic cells are capable of releasing ATP, nucleotides, and chemokines as “find-me” signals. However, unlike apoptotic cells, there are other bona fide cellular components [i.e., formylated peptides, leukotriene B4 (LTB4), hydrogen peroxide (H2O2), and complements] exposed in necrotic cells (23) (Fig. 1). Upon necrosis, formylated peptides are produced by damaged mitochondria, and act as potent chemoattractants for macrophages to necrotic sites through binding to formylated peptides receptor 1 (47–49). LTB4 is rapidly produced by necrotic leukocytes (i.e., neutrophils and macrophages) (50, 51), and acts in conjunction with formylated peptides and chemokines to recruit neutrophils (52). However, the formation of LTB4 would produce superoxide which can be converted to H2O2 by superoxide dismutase (53). Interestingly, a recent study by Yoo et al. (54) showed that H2O2 could activate Src family kinase Lyn in leukocytes, leading to enhanced chemotactic responses. Indeed, it is recognized that reduced production of H2O2 could suppress monocyte recruitment into atherosclerotic lesion (55). Moreover, using a small-molecule functional screening, Hattori et al. (56) showed that H2O2 can regulate chemotactic responses mediated by other “find-me” signals including formylated peptides, LTB4, and IL-8. Lastly, as for the complement system in necrotic cells, it is activated by cleavage of complement proteins, and then deposited onto target cells or secreted into extracellular area to interact with neighboring cells and phagocytes (23). However, current knowledge suggests that activities of complement system may vary in different tissues or different disease models (57–60). Future studies should focus on the specificity of key complement components in the efferocytosis of necrotic cells.
“Eat-me” signaling
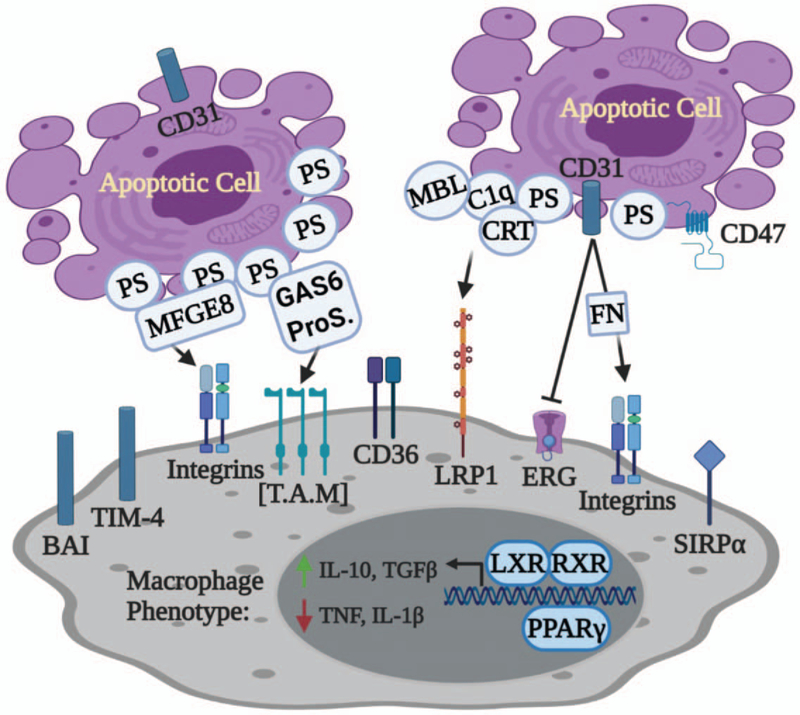
Once macrophages reach the damaged tissue, they need to distinguish dying cells from healthy cells through “eat-me” signals on the cell surface. The best characterized “eat-me” signal is the lipid mediator phosphatidylserine (PtdSer), which is normally confined to the inner leaflet of the viable cell membrane and externalized during cell death (61, 62). Up to date, there are at least 12 efferocytosis receptors identified that can bind to PtdSer directly (i.e., BAI-1, TIM-4, CD300, and Stabilin/MEGF10) or indirectly [i.e., TAM (Tyro3, Axl, Mer), tyrosine kinases, CD36, and integrins)] (Fig. 2) (21, 63). Binding of PtdSer to indirect receptors requires “bridging molecules” such as dimers of Gas6 and Protein S for MerTK (MER receptor tyrosine kinase) signaling, and MFGE8 for integrins activity (Fig. 2) (7). Recognition of PtdSer by phagocytes could trigger anti-inflammatory responses (64, 65), yet in specific context, it might promote pro-inflammatory responses (66). Other “eat-me” signals identified so far include calreticulin (CRT), ICAM-3, lipids, and modified carbohydrates (23, 67, 68).
Fig. 2. Apoptotic “eat-me” signals and their respective receptors.
The best characterized “eat-me” signal is phosphatidylserine (PtdSer, or PS), which is exposed to the outer leaflet of plasma membrane on dying cells. Macrophages can directly recognize PS through its interaction with receptors such as BAI, TIM-4, and CD36. Alternatively, PS can be bound indirectly to receptors on macrophages through “bridging proteins” such as MFGE8 for integrins, GAS6-ProS dimer for TAM tyrosine kinases. Other “eat-me” molecules include CRT and CD31. In coordination with MBL and C1q, CRT interacts with LRP1 and facilitates the recognition of PS by macrophages. In addition, CD31 activates integrins after binding to the bridging molecule fibronectin (FN). Recognition of eat-me signals switches macrophages to anti-inflammatory phenotype partly through the activation of nuclear receptors such as LXR and PPARγ. BAI indicates brain-specific angiogenesis inhibitor; C1q, complement factor C1q; CRT, calreticulin; GAS6, growth arrest-specific 6; LRP1, low-density lipoprotein (LDL) receptor-related protein 1; MBL, mannose-binding lectin; MFGE8, milk fat globule-EGF factor 8; ProS, protein S; TAM, Tyro3, Axl, Mer; TIM-4, T cell immunoglobulin mucin receptor 4.
Unlike efferocytosis of apoptotic cells, current understanding of molecular signaling involved necrotic cell uptake is limited. Several studies have shown some overlap of “eat-me” signals for necrotic cells with the signals for apoptotic cells (67). For example, PtdSer is also exposed on necrotic cells and debris through distinct mechanisms. Nonetheless, necrotic cells do express unique “eat-me” signals such as deposition of complement components, and translocation of Annexin A1 to the plasma membrane to enhance phagocytic uptake (67). As apoptotic cells enter late apoptotic or secondary necrotic stages, complement components are strongly induced to enhance their recognition by phagocytes (69). A study by Gaipl et al. (70) showed that C1a, C3b, and C4 bound weakly to irradiated lymphocytes undergoing apoptosis; however, as these lymphocytes persisted into secondary necrosis, these complement components bound to them with significantly greater affinity. Such observations suggest that deposition of complement components not only promotes necrotic cell removal, but also acts as an alternative mechanism to remove late apoptotic cells and debris. With regard to Annexin A1, it was previously reported to translocate to apoptotic cell surface (71, 72). However, Blume et al. (73) recently showed that Annexin A1 was externalized only on secondary necrotic cells, but rarely translocated to surface of primary apoptotic cells. These observations suggest that Annexin A1 may be the hallmark for secondary necrosis. Indeed, Annexin A1 has later been shown to act as a bridging molecule for phagocytes to recognize PtdSer on the cell membrane of necrotic cells (74). Importantly, other than promoting efferocytosis of dying cells, Annexin A1 is capable of suppressing the production of proinflammatory cytokines by macrophages after ingestion of necrotic cells (74).
Digestion signaling
Engulfment of dying cells or debris leads to excessive accumulation of cellular materials such as proteins, lipids, nucleotides, and carbohydrates, if not deposed properly, they could lead to inflammatory responses or autoimmunity. Therefore, postengulfment signaling to digest and efflux these materials is needed. Upon recognition by phagocytes, actin filaments are activated and orchestrated by Rac1 to form early phagosome, which contains Rab5 that is critical for the phagosome development (68, 75). Nonetheless, it remains unresolved as how Rab5 is recruited to phagosome. As early phagosome matures and transitions to late phagosome, Rab5 protein is diminished with concomitant acquisition of another GTPase Rab7 (68), which can interact with lysosome membrane proteins LAMP1 and LAMP2 to facilitate fusion of late endosome with lysosome (76). Additionally, such direct fusion requires intact microtubules and is coordinated by a Ca2+-dependent SNARE complex, composed of VAMP7 and syntaxin7 (68, 77). Ultimately, this leads to the formation of phagolysosome in which the engulfed cargoes are hydrolyzed and degraded (78). On the other hand, once internalized, lipids on phagosome membrane can be conjugated with LC3-family protein complex that contains Rubicon, VPS34, Beclin1, UVRAG, and VPS15, a process called LC3-associated phagocytosis (LAP) (79). These phagosomes are referred to as LAPosomes, which can readily fuse with lysosomes to facilitate the hydrolytic degradation of the cargoes (80).
Consequently, macrophages are overloaded with macromolecular constituents, which in turn activate multiple metabolic sensing pathways. For example, excess intracellular cholesterol could activate peroxisome proliferator-activated receptor (PPAR), retinoid X receptor, and liver X receptor (LXR) families of nuclear receptors, which are key mediators for cholesterol efflux through upregulation of ABCA1 and ABCG1 (81, 82). Regarding phagocytosed chromosomal DNA, it is degraded by DNase II within lysosomes of macrophages (83). Importantly, activation of certain metabolic sensing pathways could enhance macrophage efferocytosis through upregulation of engulfment-related genes such as PtdSer-binding soluble proteins and receptors (i.e., CD36, MerTK) (84). In addition to augmenting efferocytosis, many nuclear receptors can modulate inflammation via increasing the production of various anti-inflammatory mediators (i.e., IL-10, TGFβ, and lactate), whereas macrophages with LXR or PPARδ deficiency produce more TNF-α and IL-12 when treated with apoptotic cells (84–86). Furthermore, Yamaguchi et al. (87) observed that stimulating macrophages with apoptotic cells increased gene expression of another nuclear receptor Nr4a1, resulting in anti-inflammatory properties. Notably, Nr4a1 can be activated by adenosine, a prominent “find-me” signaling molecule (88). Put together, all these findings indicate that nuclear receptors can potentially be activated at different stages of efferocytosis, which further ensure adequate generation of pro-resolving signals.
IMPACT OF MACROPHAGE EFFEROCYTOSIS ON HEART DEVELOPMENT
During cardiac development, failure to remove apoptotic cells in a timely manner may result in fetal congenital heart block (CHB). In fact, immunohistochemical analysis of heart samples from fetuses with CHB revealed that apoptosis signal was more than 30-fold higher in septal tissues (89, 90). Of note, human and rodent fetal cardiomyocytes may act as nonprofessional phagocytes to engulf neighboring apoptotic cells (91, 92). However, very little is known about the function of professional phagocytes such as macrophages during various cardiac developmental stages. Leid et al. (93) recently observed that primitive yolk sac-derived macrophages became apparent in the heart at E11.5, which are characterized by low expression of CCR2 and MHC-II (CCR2−MHC-IIlow) on cell surface. This subset of macrophages plays essential roles in coronary development and maturation through releasing insulin-like growth factor 1 and 2 starting at E13.5 to E14.5 (93). During this developmental stage, fetal monocyte-derived macrophages (CCR2+MHC-IIlow) are also recruited to the heart (93). Nonetheless, this subset of macrophages seems dispensable for cardiac development, and their function in heart development is currently unknown. Intriguingly, Zhao and Rivkees (94) showed that, in heart samples from rats at E16, macrophages were noticed during engulfment of dying mesenchymal cells, suggesting that macrophage efferocytosis may modulate early cardiac development. Several recent studies further indicate that, throughout the first 2 weeks of neonatal heart development, both primitive and definitive CCR2− macrophages with low expression of MHC-II are present (18, 95). Besides potential roles in facilitating heart development and maturation, these CCR2− macrophages are also pivotal regulators for cardiac tissue repair in neonatal hearts (14, 19, 96).
EFFECTS OF MACROPHAGE EFFEROCYTOSIS ON ADULT HEART HOMEOSTASIS
The number of cardiomyocytes is established perinatally and remains virtually constant throughout life. The turnover of cardiomyocytes is estimated to be less than 1% per year in adulthood. In contrast, the exchange rates for endothelial cells and mesenchymal cells (including fibroblasts and smooth muscle cells) are roughly 20% and 5% per year, respectively (97, 98). Therefore, efficient removal of unwanted cells or debris is essential to maintain homeostasis and avoid inciting inflammation.
In adult human and mouse hearts, macrophages can be classified into three distinct subsets: CCR2−MHC-IIlow; CCR2−MHC-IIhigh; and CCR2+MHC-IIhigh (18, 99). The first two populations (CCR2−) are referred to as tissue-resident macrophages that are derived from embryonic precursors and maintained through local proliferation. They are the first responders to damaged tissue by releasing cytokines and chemokines for recruiting granulocytes from blood (100). In addition, they are actively involved in resolving inflammation and facilitate the reparative tissue remodeling processes (18, 99). More importantly, tissue resident CCR2− macrophages display enhanced phagocytic capacity for dying cardiomyocytes when compared with CCR2+ macrophages (99). In vitro studies further demonstrated that CCR2−MHC-IIlow macrophages are the most efficient population in eliminating apoptotic cells or necrotic cell debris (18, 101). In the contrary, CCR2+MHC-IIhigh macrophages are exclusively derived from circulating monocytes and maintained through differentiation and proliferation of infiltrated monocytes (18, 19). They are generally considered as pro-inflammatory population by activation of NLRP3 and IL-1β secretion, and subsequently contribute to adverse cardiac remodeling (18, 19, 99). Furthermore, sufficient efferocytosis is particularly important in the elderly population, as inadequate removal of apoptotic or senescent cells can lead to non-resolving inflammation, delayed or maladaptive cardiac repair, and thereby increases risk for heart failure (102, 103).
MACROPHAGE EFFEROCYTOSIS IN CARDIAC INJURY AND REPAIR
MerTK in macrophage efferocytosis
One of the prominent functions of cardiac macrophages is the clearance of dying/dead cells and extracellular matrix. Defective efferocytosis by macrophages in both neonatal and adult hearts can lead to impaired tissue remodeling and function, particularly following insults from myocardial infarction (MI) and/or reperfusion (14, 104, 105). Up to date, MerTK is the best-characterized efferocytosis receptor on macrophages during myocardial infarction. It can recognize PtdSer on dying/dead cells through binding to “bridging” molecules Gas6 and Protein S (7), which could cause receptor dimerization with MerTK itself or other tyrosine kinase receptors including Tyro3 and Axl, leading to activation of Rac-1 and engulfment of target cells/debris (106). Wan et al. (107) recently found that the expression of MerTK was upregulated in left ventricle after MI, peaking at day 7 post-surgery in the ischemic zone. Furthermore, using MerTK-knockout mouse model, these authors observed that apoptotic cardiomyocytes were accumulated to a greater degree when comparing to WT hearts after MI (107). Importantly, defective efferocytosis at early stage of MI promoted larger scar formation accompanied by exacerbated cardiac dysfunction at long term (28 days after MI) (107). Of note, an inactivated form of MerTK, known as solMER, was detected in the heart 5 days after MI (107). Interestingly, a recent study by Suresh et al. showed that, in both human diabetic hearts and high glucose-treated macrophages, expression levels of miR-126 were reduced with a corresponding upregulation of ADAM9 (a dis-integrin and metalloproteinase), compared with normal controls (108). Notably, ADAM9 could induce proteolytic cleavage of MerTK, leading to higher levels of solMer in macrophages, and consequently impaired efferocytosis of apoptotic/necrotic cardiomyocytes (108). Therefore, restoring MerTK activity or prevention of MerTK cleavage may be of great therapeutic value to reduce cardiac damage from MI or metabolic diseases.
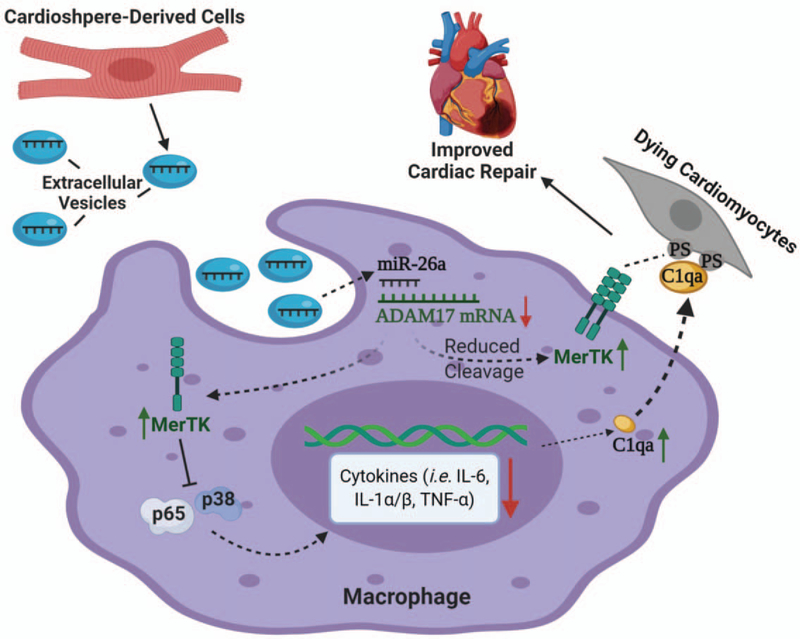
Similarly, a recent study by de Couto et al. (104) demonstrated that expression of MerTK is necessary for enhanced efferocytosis and cardio-protection. These authors first treated bone marrow-derived macrophages (MΦ) with extracellular vesicles isolated from cardiosphere-derived cells (MΦ-CDCev). By using both in vitro and in vivo efferocytosis assays, they showed that MΦ-CDCev could enhance clearance of apoptotic/necrotic cells, resulting in reduced infarct size after MI (104). More importantly, such protective effects were diminished when applied to MerTK-knockout mice (104). Further investigation revealed that CDCev treatment upregulated miR-26a, which subsequently suppressed the expression of ADAM17 (another disintegrin and metalloproteinase that can cleave MerTK) and reduced the cleavage of MerTK (104). Meanwhile, transfer of CDCev increased cellular content of complement protein C1qa in macrophages, which could facilitate the bridging of macrophages with dying cells and thus stimulated efferocytosis (Fig. 3) (104). Collectively, these findings mentioned above have clearly demonstrated the importance of effective efferocytosis and the therapeutic potential of MerTK as a candidate to ameliorate cardiac injury. Nonetheless, future studies focusing on MerTK-independent efferocytosis pathways should be warranted to provide further insight into this dynamic process.
Fig. 3. Transfer of extracellular vesicles containing miR-26a from cardiosphere-derived cells to macrophages enhances efferocytosis of dying cardiomyocytes in MI models.
Extracellular vesicles released by cardiosphere-derived cells (CDCev) transfer miR-26a to targeting macrophages, leading to inhibition of ADAM17 mRNA translation. Consequently, ADAM17-mediated cleavage of MerTK is reduced, resulting in increased levels of active MerTK. In addition, CDCev also upregulate complement factor C1q in macrophages. Thus, increased levels of MerTK and C1q promote the recognition of “eat-me” signals and stimulate the efferocytosis of dying cardiomyocytes. Furthermore, sustained MerTK inhibits pro-inflammatory responses (i.e., reduced levels of IL-6, IL-1α/β, and TNFα) in macrophages via suppression of NF-κB and MAPK pathways. Therefore, augmented efferocytosis together with reduced inflammation results in improved cardiac repair after MI injury.
Efferocytosis in resolving cardiac inflammation
Upon cardiac injury, influx of neutrophils occurs prior to monocyte/macrophage infiltration to damaged tissue (109, 110). As terminally differentiated cells, neutrophils start undergoing apoptosis shortly after reaching the infarcted myocardium and disintegrate after 48 h (111, 112). Consequently, they are capable of releasing phagocytic signals such as nucleotides (“find-me”) and PtdSer (“Eat-me”) that can be readily recognized by macrophages (111–113). It is now well appreciated that enhanced efferocytosis by macrophages can activate anti-inflammatory program by downregulating proinflammatory cytokines and increasing the production of proresolving factors such as IL-10 and TGF-β (114). In the presence of proinflammatory stimuli (i.e. cytokines, TLR ligands), TGF-β can inhibit inflammatory gene synthesis in macrophages through the activation of Smad3 pathway (115, 116). Interestingly, efferocytic macrophages are able to directly and rapidly activate Smad3 without active TGF-β release; and Smad3-deficient macrophages exhibited blunted efferocytosis capacity with concomitant defective anti-inflammatory actions, as evidenced by marked increase in the expression of IL-1β, TNF-α, and CCL2 (117).
It is important to mention here that after initiating the recruitment of leukocytes, tissue resident macrophages diminish within 2 h of injury, and the cardiac pool is primarily replenished by circulating monocyte-derived macrophages in two sequential phases (118, 119). During the inflammatory phase (first 5 days post-MI), CCR2+Ly6Chigh monocytes accumulate in the heart in response to CCL2/MCP-1 (“find-me”), and they display pro-inflammatory phenotype by secreting cytokines (i.e., TNF-α and IL-6) (120, 121). During the reparative phase, Ly6Chigh monocytes/macrophages differentiate to Ly6Clow macrophages to increase efferocytosis and reduce inflammation through the Nr4a1-mediated signaling pathway (120). Recently, Marinković et al. (122) showed that extended blockade of S100A9 hindered the trafficking of monocytes from spleen to the heart in mice after MI, and this was accompanied with impaired transition of pro-inflammatory Ly6Chigh phenotype to anti-inflammatory Ly6ClowMerTKhi phenotype, resulting in insufficient efferocytosis and stronger inflammatory responses. This interference of macrophage phenotype transition was due to the downregulation and inhibition of Nr4a1 after blocking S100A9 (122). Furthermore, Nr4a1 can positively regulate MerTK expression by binding directly to the gene regulatory elements (123, 124). Interestingly, Nr4a1, as a potent anti-inflammatory mediator, is necessary for phagocyte survival and can be induced by efferocytosis signaling such as adenosine (“find-me”) or activation of CD36 (“Eat-me”). Supportively, it has been demonstrated that Nr4a1-deficient macrophages produce more nitric oxide and pro-inflammatory cytokines with impaired phagocytic capacity (125, 126). Therefore, it is reasonable to speculate that adequate efferocytosis is a critical prerequisite for timely resolution of inflammation, and many pathways in efferocytosis and anti-inflammatory programs are intertwined that cooperatively modulate macrophage functions in the heart.
Effects of macrophage efferocytosis on cardiac angiogenesis
Efferocytosis may also participate in post-MI angiogenesis and vascular bed regeneration through either physically mediated anastomosis or secretion of paracrine factors (127–129). During transition from pro-inflammatory to reparative phenotype, macrophages in the myocardium release numerous growth factors such as TGF-β and VEGF (130), which are well known for promoting angiogenesis and vascular formation during reparative phase post-MI (130, 131). Interestingly, the expression of TGF-β and VEGF has been reported to be regulated by MerTK and MFGE8 (101, 132, 133). Moreover, mice with macrophage-specific knockout of TGF-β receptor II (TGF-βRII) or VEGF exhibited a blunted reparative function of macrophages, leading to impaired cardiac function and angiogenesis (133, 134). By contrast, stimulation with TGF-β ligand or overexpression of VEGF increased recruitment of monocytes to injured site and promoted pro-angiogenic phenotype (130, 135). Additionally, Chen et al. (117) showed that TGF-β-induced Smad3 signaling was required for proper cardiac efferocytosis function. They observed that expression levels of genes involved “eat-me” signaling (i.e., MFGE8, IL-10, TGF-β1, and VEGF) were reduced in Smad3-null bone marrow-derived macrophages. Mice with myeloid cell-specific knockout of Smad3, after 28 days of permanent coronary occlusion, showed increased mortality and worsened cardiac dysfunction, which was associated with defective macrophage efferocytosis, leading to scar expansion and cardiomyocyte death of the border zone (117).
Macrophage efferocytosis signals in cardiac repair
Pro-inflammatory macrophages clear injury site through efferocytosis and secretion of proteases (i.e., MMP2 and MMP9) (136). During the final stages of cardiac repairing and remodeling, timely transition of these macrophages toward reparative phenotype and proper neutralization of proteases are vital in preventing adverse cardiac remodeling or rupture (136). On the other hand, it has been shown that suppression of macrophage transition toward pro-inflammatory phenotype through inhibition of MAPK and NF-κB pathways could attenuate cardiomyocyte death upon MI injury (137). In addition, macrophage class A scavenger receptor (SR-A) is widely known as “eat-me” signal in efferocytosis of dying cells (138–140). Nonetheless, loss of SR-A is reported to upregulate TNF-α and increase proteolytic activity of MMP9, ultimately leading to post-MI cardiac rupture (141). Indeed, MMP9 can target factors involved in efferocytosis signaling including chemokines of CXCL family, CD36 (“eat-me”) and Thbs-1 (pro-resolving) (142); and depletion of MMP9 could promote CD36-dependent macrophage efferocytosis and reduce risk of cardiac rupture after MI injury (143, 144). However, effects of SR-A are dependent on the type and duration of cardiac injury (141, 145, 146). For example, SR-A knockout mice subjected to cardiac ischemia/reperfusion showed significantly exacerbated cardiac inflammation and cardiomyocyte death (146). By contrast, SR-A-deficient mice with permanent LAD occlusion (MI model) showed improved survival and reduced inflammation and cardiac rupture after 21 days (137, 141).
Regarding whether efferocytosis of collagen would promote extracellular matrix deposition, Vagnozzi et al. recently showed that phagocytic macrophages with increased expression of CX3CR1 (“find-me”) could alter cardiac fibroblast activity, leading to reduced extracellular matrix content in the border zone and enhanced cardiac contractility (147). Furthermore, these authors demonstrated that, 3 days after injecting bone marrow-derived mononucleated cells into the heart after ischemia/reperfusion, macrophage subtypes were shifted from mainly CCR2−CX3CR1+ phenotype observed in basal condition to a mixed population of CCR2+ and CCR2+CX3CR1+ macrophages (147). These data suggest that the pro-inflammatory population is gradually acquiring a reparative phenotype. Likewise, anti-inflammatory macrophages expressing mannose receptor (CD206+) also contribute to the efferocytosis of collagen and possibly involve new extracellular matrix deposition, as evidenced by that depletion of CD206+ macrophages in MI triggers substantial decrease in collagen contents (148, 149). More importantly, crosstalk between fibroblasts and phagocytes could affect the efficiency of efferocytosis and reparative process. For example, during acute injury from autoimmune myocarditis, activated fibroblasts are able to not only inhibit Ly6Clow monocyte differentiation to reparative macrophages through upregulation of IL-17A, but also promote shedding of MerTK on Ly6Chigh macrophages, leading to an overall pro-inflammatory phenotype with impaired efferocytosis activity (150). Moreover, IL-17A may act as autocrine for stimulating cardiac fibroblasts to boost secretion of GM-CSF, which in turn direct Ly6Chigh macrophages toward more proinflammatory phenotype in the dilated cardiomyopathy model (151). Notably, reparative macrophages could release a large amount of TGFβ1, which drives the phenotypic transformation of homeostatic fibroblasts to myofibroblasts that have superior capacity to produce matrix components (136, 152).
“DON'T EAT-ME” SIGNALS IN THE HEART
Given the long lifespan and the extremely low cellular turnover of cardiomyocytes, it is plausible to speculate that anti-phagocytic (or “don’t eat-me” signaling) may play important roles in repelling macrophages. Aging mice with thrombospondin-2 (a CD47 ligand) deficiency exhibit impaired cardiomyocyte survival via suppression of Akt pathway, which is accompanied with increased cardiac inflammation and fibrosis, leading to dilated cardiomyopathy (153). These findings suggest that “don’t eat-me” signaling is important to maintain proper cardiac homeostasis. Nonetheless, it remains incompletely understood whether antiphagocytic signaling directly participates in dying cardiac cell removal. Recently, several studies have indicated that CD47 expression is highly upregulated in apoptotic neonatal cardiomyocytes and in hearts after MI, and activation of CD47 with thrombospondin-1 upregulates HDAC3 via Ca2+-CaMKII signaling pathway, resulting in myocyte hypertrophy (154–156). Furthermore, blocking with anti-CD47 antibody could suppress hypertrophy but enhance clearance of apoptotic myocytes and resolution of inflammation, leading to reduced infarct size and improved contractile function (154, 155). Therefore, along with other standards of treatment regimes, early targeting of CD47 in the heart may provide better clinical outcome by enhancing efferocytosis of damaged cardiac cells, and thereby improving wound repair in ischemic hearts. Further investigation to determine the doses and timing of CD47 antibodies should be warranted to prevent collateral efferocytosis on neighboring viable cells.
CONCLUSION AND FUTURE DIRECTION
Despite the development and improvement of many clinical treatments for patients with MI and heart failure, the incidents of new and recurrent heart failure, as well as mortality, are still growing worldwide. New strategic approaches aiming to halt aberrant inflammatory signaling and improve cardiac repair are gaining more attention due to growing number of patients who have become intolerant or show serious adverse effects of existing treatment options. With fast advancing technologies in gene-editing and Flow Cytometry, a new era has emerged in which diverse macrophage populations with distinct origins and function are being characterized. Enhanced appreciation of these macrophages is recognized as their therapeutic potentials have been explored vastly in different animal and disease models. Nonetheless, an important question remains: whether human heart contains similar populations of macrophages as animal heart? Although studies have shown that, similar to mouse models, cardiac macrophages can be partitioned into different groups using CCR2 and MHC-II markers in human failing hearts (18, 119), the exact roles of these divergent macrophages remain to be determined, and the underlying molecular mechanisms governing the activity and function of these macrophages need to be investigated.
Up to date, overall benefits of targeting inflammation remain inconclusive, based on the results from different clinical trials. For example, the Cardiovascular Inflammation Reduction Trial reported that effects of low-dose methotrexate in patients with stable atherosclerosis did not differ from placebo treatments (157). However, according to the results from Canakinumab Anti-Inflammatory Thrombosis Outcomes Study, targeting IL-1β pathway with Canakinumab was able to not only lower the rate of recurrent cardiovascular events without affecting lipids (158), but also lower heart failure-related hospitalization and mortality in patients with previous myocardial infarction (159). Given that the execution of efferocytosis is intertwined with modulating inflammatory responses, targeting the processes associated with efferocytosis may guide the treatment on cardiac inflammation, which can be highly context-specific, as different types and stages of cardiac injury may present distinct microenvironmental cues to fine-tune the activity of macrophages. For example, deficiency of SR-A elicits protective effects during acute ischemia/reperfusion injury, whereas it promotes adverse cardiac remodeling in a long-term cardiac ischemic model (160). In addition, macrophage efferocytosis requires both the inhibition of “don’t eat-me” signals and presentation of “find-me” and “eat-me” signals. Therefore, multiple levels of check and balance to tightly regulate the targeting of efferocytosis could minimize the off-target effects. In conclusion, development of therapeutic approaches to manipulate efferocytosis represents a promising strategy to properly control cardiac inflammation and promote cardiac repair in aging individuals as well as patients with various cardiac injuries.
Acknowledgments
Research in Fan lab was supported by American Heart Association (AHA) Established Investigator Award [17EIA33400063 to G-CF], National Institute of Health grants [GM-126061 and GM-132149 to Dr G-CF], AHA Pre-doctoral Fellowship [18PRE33960576 to YL].
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Arandjelovic S, Ravichandran KS: Phagocytosis of apoptotic cells in homeostasis. Nat Immunol 16:907–917, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bianconi E, Piovesan A, Facchin F, Beraudi A, Casadei R, Frabetti F, Vitale L, Pelleri MC, Tassani S, Piva F, et al. : An estimation of the number of cells in the human body. Ann Hum Biol 40:463–471, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Vandivier RW, Henson PM, Douglas IS: Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest 129:1673–1682, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Ravichandran KS, Lorenz U: Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol 7:964–974, 2007. [DOI] [PubMed] [Google Scholar]
- 5.deCathelineau AM, Henson PM: The final step in programmed cell death: phagocytes carry apoptotic cells to the grave. Essays Biochem 39:105–117, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Henson PM, Hume DA: Apoptotic cell removal in development and tissue homeostasis. Trends Immunol 27:244–250, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Morioka S, Maueröder C, Ravichandran KS: Living on the edge: efferocytosis at the interface of homeostasis and pathology. Immunity 50:1149–1162, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yurdagul A Jr, Subramanian M, Wang X, Crown SB, Ilkayeva OR, Darville L, Kolluru GK, Rymond CC, Gerlach BD, Zheng Z, et al. : Macrophage metabolism of apoptotic cell-derived arginine promotes continual efferocytosis and resolution of injury. Cell Metab 31:518–533, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henson PM: Cell removal: efferocytosis. Annu Rev Cell Dev Biol 33:127–144, 2017. [DOI] [PubMed] [Google Scholar]
- 10.Rabinovitch M: Professional and non-professional phagocytes: an introduction. Trends Cell Biol 5:85–87, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Cummings RJ, Barbet G, Bongers G, Hartmann BM, Gettler K, Muniz L, Furtado GC, Cho J, Lira SA, Blander JM: Different tissue phagocytes sample apoptotic cells to direct distinct homeostasis programs. Nature 539:565–569, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seeberg JC, Loibl M, Moser F, Schwegler M, Buttner-Herold M, Daniel C, Engel FB, Hartmann A, Schlötzer-Schrehardt U, Goppelt-Struebe M, et al. : Non-professional phagocytosis: a general feature of normal tissue cells. Sci Rep 9:11875, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, et al. : Revisiting cardiac cellular composition. Circ Res 118:400–409, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN: Macrophages are required for neonatal heart regeneration. J Clin Invest 124:1382–1392, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Furth R, Cohn ZA: The origin and kinetics of mononuclear phagocytes. J Exp Med 128:415–435, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epelman S, Liu PP, Mann DL: Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol 15:117–129, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sieweke MH, Allen JE: Beyond stem cells: self-renewal of differentiated macrophages. Science 342:1242974, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, et al. : Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40:91–104, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL: Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci U S A 111:16029–16034, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JM: Red blood cell population dynamics. Clin Lab Med 35:43–57, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott MR, Koster KM, Murphy PS: Efferocytosis signaling in the regulation of macrophage inflammatory responses. J Immunol 198:1387–1394, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tajbakhsh A, Rezaee M, Barreto GE, Moallem SA, Henney NC, Sahebkar A: The role of nuclear factors as “Find-Me”/alarmin signals and immunostimulation in defective efferocytosis and related disorders. Int Immunopharmacol 80:106134, 2020. [DOI] [PubMed] [Google Scholar]
- 23.Westman J, Grinstein S, Marques PE: Phagocytosis of necrotic debris at sites of injury and inflammation. Front Immunol 10:3030, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elliott MR, Ravichandran KS: The dynamics of apoptotic cell clearance. Dev Cell 38:147–160, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, et al. : Pannexin 1 channels mediate ’find-me’ signal release and membrane permeability during apoptosis. Nature 467:863–867, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, et al. : Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461:282–286, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marques-da-Silva C, Burnstock G, Ojcius DM, Coutinho-Silva R: Purinergic receptor agonists modulate phagocytosis and clearance of apoptotic cells in macrophages. Immunobiology 216:1–11, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Joós G, Jákim J, Kiss B, Szamosi R, Papp T, Felszeghy S, Sághy T, Nagy G, Szondy Z: Involvement of adenosine A3 receptors in the chemotactic navigation of macrophages towards apoptotic cells. Immunol Lett 183:62–72, 2017. [DOI] [PubMed] [Google Scholar]
- 29.Ohta A, Sitkovsky M: Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 414:916–920, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Haskó G, Cronstein B: Regulation of inflammation by adenosine. Front Immunol 4:85, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cronstein BN, Sitkovsky M: Adenosine and adenosine receptors in the pathogenesis and treatment of rheumatic diseases. Nat Rev Rheumatol 13:41–51, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Truman LA, Ford CA, Pasikowska M, Pound JD, Wilkinson SJ, Dumitriu IE, Melville L, Melrose LA, Ogden CA, Nibbs R, et al. : CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood 112:5026–5036, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Sokolowski JD, Chabanon-Hicks CN, Han CZ, Heffron DS, Mandell JW: Fractalkine is a “find-me” signal released by neurons undergoing ethanol-induced apoptosis. Front Cell Neurosci 8:360, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miksa M, Amin D, Wu R, Ravikumar TS, Wang P: Fractalkine-induced MFGE8 leads to enhanced apoptotic cell clearance by macrophages. Mol Med 13:553–560, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noda M, Doi Y, Liang J, Kawanokuchi J, Sonobe Y, Takeuchi H, Mizuno T, Suzumura A: Fractalkine attenuates excito-neurotoxicity via microglial clearance of damaged neurons and antioxidant enzyme heme oxygenase-1 expression. J Biol Chem 286:2308–2319, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morganti JM, Nash KR, Grimmig BA, Ranjit S, Small B, Bickford PC, Gemma C: The soluble isoform of CX3CL1 is necessary for neuroprotection in a mouse model of Parkinson’s disease. J Neurosci 32:14592–14601, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cullen SP, Henry CM, Kearney CJ, Logue SE, Feoktistova M, Tynan GA, Lavelle EC, Leverkus M, Martin SJ: Fas/CD95-induced chemokines can serve as “find-me” signals for apoptotic cells. Mol Cell 49:1034–1048, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Lauber K, Bohn E, Kröber SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, et al. : Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 113:717–730, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Peter C, Waibel M, Keppeler H, Lehmann R, Xu G, Halama A, Adamski J, Schulze-Osthoff K, Wesselborg S, Lauber K: Release of lysophospholipid ’find-me’ signals during apoptosis requires the ATP-binding cassette transporter A1. Autoimmunity 45:568–573, 2012. [DOI] [PubMed] [Google Scholar]
- 40.Yvan-Charvet L, Pagler TA, Seimon TA, Thorp E, Welch CL, Witztum JL, Tabas I, Tall AR: ABCA1 and ABCG1 protect against oxidative stress-induced macrophage apoptosis during efferocytosis. Circ Res 106:1861–1869, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le LQ, Kabarowski JH, Weng Z, Satterthwaite AB, Harvill ET, Jensen ER, Miller JF, Witte ON: Mice lacking the orphan G protein-coupled receptor G2A develop a late-onset autoimmune syndrome. Immunity 14:561–571, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Kabarowski JH: G2A and LPC: regulatory functions in immunity. Prostaglandins Other Lipid Mediat 89:73–81, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weigert A, Olesch C, Brune B: Sphingosine-1-phosphate and macrophage biology-how the sphinx tames the big eater. Front Immunol 10:1706, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gude DR, Alvarez SE, Paugh SW, Mitra P, Yu J, Griffiths R, Barbour SE, Milstien S, Spiegel S: Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a “come-and-get-me” signal. FASEB J 22:2629–2638, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weigert A, Tzieply N, von Knethen A, Johann AM, Schmidt H, Geisslinger G, Brune B: Tumor cell apoptosis polarizes macrophages role of sphingosine-1-phosphate. Mol Biol Cell 18:3810–3819, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo B, Gan W, Liu Z, Shen Z, Wang J, Shi R, Liu Y, Liu Y, Jiang M, Zhang Z, et al. : Erythropoeitin signaling in macrophages promotes dying cell clearance and immune tolerance. Immunity 44:287–302, 2016. [DOI] [PubMed] [Google Scholar]
- 47.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P: Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330:362–366, 2010. [DOI] [PubMed] [Google Scholar]
- 48.Heit B, Tavener S, Raharjo E, Kubes P: An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J Cell Biol 159:91–102, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dorward DA, Lucas CD, Chapman GB, Haslett C, Dhaliwal K, Rossi AG: The role of formylated peptides and formyl peptide receptor 1 in governing neutrophil function during acute inflammation. Am J Pathol 185(5):1172–1184, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kienle K, Lämmermann T: Neutrophil swarming: an essential process of the neutrophil tissue response. Immunol Rev 273:76–93, 2016. [DOI] [PubMed] [Google Scholar]
- 51.Majumdar R, Tavakoli Tameh A, Parent CA: Exosomes mediate LTB4 release during neutrophil chemotaxis. PLoS Biol 14:e100233, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Lämmermann T, Afonso PV, Angermann BR, Wang JM, Kastenmüller W, Parent CA, Germain RN: Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498:371–375, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Demiryürek AT, Wadsworth RM: Superoxide in the pulmonary circulation. Pharmacol Ther 84:355–365, 1999. [DOI] [PubMed] [Google Scholar]
- 54.Yoo SK, Starnes TW, Deng Q, Huttenlocher A: Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature 480:109–112, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang D, Jiang X, Fang P, Yan Y, Song J, Gupta S, Schafer AI, Durante W, Kruger WD, Yang X, et al. : Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine beta-synthase-deficient mice. Circulation 120:1893–1902, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hattori H, Subramanian KK, Sakai J, Jia Y, Li Y, Porter TF, Loison F, Sarraj B, Kasorn A, Jo H, et al. : Small-molecule screen identifies reactive oxygen species as key regulators of neutrophil chemotaxis. Proc Natl Acad Sci U S A 107:3546–3551, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ehrengruber MU, Geiser T, Deranleau DA: Activation of human neutrophils by C3a and C5A. Comparison of the effects on shape changes, chemotaxis, secretion, and respiratory burst. FEBS Lett 346:181–184, 1994. [DOI] [PubMed] [Google Scholar]
- 58.Karsten CM, Laumonnier Y, Köhl J: Functional analysis of C5a effector responses in vitro and in vivo. Methods Mol Biol 1100:291–304, 2014. [DOI] [PubMed] [Google Scholar]
- 59.Marshall KM, He S, Zhong Z, Atkinson C, Tomlinson S: Dissecting the complement pathway in hepatic injury and regeneration with a novel protective strategy. J Exp Med 211:1793–1805, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang C, Wang C, Li Y, Miwa T, Liu C, Cui W, Song WC, Du J: Complement C3a signaling facilitates skeletal muscle regeneration by regulating monocyte function and trafficking. Nat Commun 8:2078, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suzuki J, Imanishi E, Nagata S: Xkr8 phospholipid scrambling complex in apoptotic phosphatidylserine exposure. Proc Natl Acad Sci U S A 113:9509–9514, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park SY, Kim IS: Engulfment signals and the phagocytic machinery for apoptotic cell clearance. Exp Mol Med 49:e331, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arienti S, Barth ND, Dorward DA, Rossi AG, Dransfield I: Regulation of apoptotic cell clearance during resolution of inflammation. Front Pharmacol 10:891, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huynh ML, Fadok VA, Henson PM: Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest 109:41–50, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bagalkot V, Deiuliis JA, Rajagopalan S, Maiseyeu A: “Eat me” imaging and therapy. dv Drug Deliv Rev 99:2–11, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ocanña-Guzman R, Torre-Bouscoulet L, Sada-Ovalle I: TIM-3 regulates distinct functions in macrophages. Front Immunol 7:229, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boada-Romero E, Martinez J, Heckmann BL, Green DR: The clearance of dead cells by efferocytosis. Nat Rev Mol Cell Biol 21:398–414, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosales C, Uribe-Querol E: Phagocytosis: a fundamental process in immunity. Biomed Res Int 2017:9042851, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trouw LA, Blom AM, Gasque P: Role of complement and complement regulators in the removal of apoptotic cells. Mol Immunol 45(5):1199–1207, 2008. [DOI] [PubMed] [Google Scholar]
- 70.Gaipl US, Kuenkele S, Voll RE, Beyer TD, Kolowos W, Heyder P, Kalden JR, Herrmann M: Complement binding is an early feature of necrotic and a rather late event during apoptotic cell death. Cell Death Differ 8:327–334, 2001. [DOI] [PubMed] [Google Scholar]
- 71.Arur S, Uche UE, Rezaul K, Fong M, Scranton V, Cowan AE, Mohler W, Han DK: Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev Cell 4:587–598, 2003. [DOI] [PubMed] [Google Scholar]
- 72.Fan X, Krahling S, Smith D, Williamson P, Schlegel RA: Macrophage surface expression of annexins I and II in the phagocytosis of apoptotic lymphocytes. Mol Biol Cell 15:2863–2872, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blume KE, Soeroes S, Waibel M, Keppeler H, Wesselborg S, Herrmann M, Schulze-Osthoff K, Lauber K: Cell surface externalization of annexin A1 as a failsafe mechanism preventing inflammatory responses during secondary necrosis. J Immunol 183:8138–8147, 2009. [DOI] [PubMed] [Google Scholar]
- 74.Blume KE, Soeroes S, Keppeler H, Stevanovic S, Kretschmer D, Rautenberg M, Wesselborg S, Lauber K: Cleavage of annexin A1 by ADAM10 during secondary necrosis generates a monocytic “find-me” signal. J Immunol 188:135–145, 2012. [DOI] [PubMed] [Google Scholar]
- 75.Vieira OV, Bucci C, Harrison RE, Trimble WS, Lanzetti L, Gruenberg J, Schreiber AD, Stahl PD, Grinstein S: Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase. Mol Cell Biol 23:2501–2514, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huynh KK, Eskelinen EL, Scott CC, Malevanets A, Saftig P, Grinstein S: LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J 26:313–324, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fairn GD, Grinstein S: How nascent phagosomes mature to become phagolysosomes. Trends Immunol 33:397–405, 2012. [DOI] [PubMed] [Google Scholar]
- 78.Xu H, Ren D: Lysosomal physiology. Annu Rev Physiol 77:57–80, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, et al. : Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 450:1253–1257, 2007. [DOI] [PubMed] [Google Scholar]
- 80.Martinez J, Malireddi RK, Lu Q, Cunha LD, Pelletier S, Gingras S, Orchard R, Guan JL, Tan H, Peng J, et al. : Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat Cell Biol 17:893–906, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Wang B, Tontonoz P: Liver X receptors in lipid signalling and membrane homeostasis. Nat Rev Endocrinol 14:452–463, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schulman IG: Liver X receptors link lipid metabolism and inflammation. FEBS Lett 591:2978–2991, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kawane K, Ohtani M, Miwa K, Kizawa T, Kanbara Y, Yoshioka Y, Yoshikawa H, Nagata S: Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature 443:998–1002, 2006. [DOI] [PubMed] [Google Scholar]
- 84.A-Gonzalez N, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, Deniz J, Ramirez C, Díaz M, Gallardo G, et al. : Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity 31:245–258, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morioka S, Perry JSA, Raymond MH, Medina CB, Zhu Y, Zhao L, Serbulea V, Onengut-Gumuscu S, Leitinger N, Kucenas S, et al. : Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release. Nature 563:714–718, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mukundan L, Odegaard JI, Morel CR, Heredia JE, Mwangi JW, Ricardo-Gonzalez RR, Goh YP, Eagle AR, Dunn SE, Awakuni JU, et al. : PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat Med 15:1266–1272, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamaguchi H, Maruyama T, Urade Y, Nagata S: Immunosuppression via adenosine receptor activation by adenosine monophosphate released from apoptotic cells. Elife 3:e02172, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ipseiz N, Uderhardt S, Scholtysek C, Steffen M, Schabbauer G, Bozec A, Schett G, Krönke G: The nuclear receptor Nr4a1 mediates anti-inflammatory effects of apoptotic cells. J Immunol 192:4852–4858, 2014. [DOI] [PubMed] [Google Scholar]
- 89.Tran HB, Macardle PJ, Hiscock J, Cavill D, Bradley J, Buyon JP, Gordon TP: Anti-La/SSB antibodies transported across the placenta bind apoptotic cells in fetal organs targeted in neonatal lupus. Arthritis Rheum 46:1572–1579, 2002. [DOI] [PubMed] [Google Scholar]
- 90.Clancy RM, Kapur RP, Molad Y, Askanase AD: Buyon JP. immunohistologic evidence supports apoptosis, IgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum 50:173–182, 2004. [DOI] [PubMed] [Google Scholar]
- 91.Wood W, Turmaine M, Weber R, Camp V, Maki RA, McKercher SR, Martin P: Mesenchymal cells engulf and clear apoptotic footplate cells in macrophage-less PU.1 null mouse embryos. Development 127:5245–5252, 2000. [DOI] [PubMed] [Google Scholar]
- 92.Clancy RM, Neufing PJ, Zheng P, O’Mahony M, Nimmerjahn F, Gordon TP, Buyon JP: Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. J Clin Invest 116:2413–2422, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leid J, Carrelha J, Boukarabila H, Epelman S, Jacobsen SE, Lavine KJ: Primitive embryonic macrophages are required for coronary development and maturation. Circ Res 118:1498–1511, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao Z, Rivkees SA: Programmed cell death in the developing heart: regulation by BMP4 and FGF2. Dev Dyn 217:388–400, 2000. [DOI] [PubMed] [Google Scholar]
- 95.Epelman S, Lavine KJ, Randolph GJ: Origin and functions of tissue macrophages. Immunity 41:21–35, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Godwin JW, Pinto AR, Rosenthal NA: Chasing the recipe for a pro-regenerative immune system. Semin Cell Dev Biol 61:71–79, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, et al. : Dynamics of cell generation and turnover in the human heart. Cell 161:1566–1575, 2015. [DOI] [PubMed] [Google Scholar]
- 98.Lázár E, Sadek HA, Bergmann O: Cardiomyocyte renewal in the human heart: insights from the fall-out. Eur Heart J 38:2333–2342, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bajpai G, Schneider C, Wong N, Bredemeyer A, Hulsmans M, Nahrendorf M, Epelman S, Kreisel D, Liu Y, Itoh A, et al. : The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med 24:1234–1245, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Soehnlein O, Lindbom L: Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol 10:427–439, 2010. [DOI] [PubMed] [Google Scholar]
- 101.DeBerge M, Yeap XY, Dehn S, Zhang S, Grigoryeva L, Misener S, Procissi D, Zhou X, Lee DC, Muller WA, et al. : MerTK cleavage on resident cardiac macrophages compromises repair after myocardial ischemia reperfusion injury. Circ Res 121:930–940, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen W, Frangogiannis NG: The role of inflammatory and fibrogenic pathways in heart failure associated with aging. Heart Fail Rev 15:415–422, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nathan C, Ding A: Nonresolving inflammation. Cell 140:871–882, 2010. [DOI] [PubMed] [Google Scholar]
- 104.de Couto G, Jaghatspanyan E, DeBerge M, Liu W, Luther K, Wang Y, Tang J, Thorp EB, Marbán E: Mechanism of enhanced MerTK-dependent macrophage efferocytosis by extracellular vesicles. Arterioscler Thromb Vasc Biol 39:2082–2096, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Frantz S, Hofmann U, Fraccarollo D, Schäfer A, Kranepuhl S, Hagedorn I, Nieswandt B, Nahrendorf M, Wagner H, Bayer B, et al. : Monocytes/macrophages prevent healing defects and left ventricular thrombus formation after myocardial infarction. FASEB J 27:871–881, 2013. [DOI] [PubMed] [Google Scholar]
- 106.Myers KV, Amend SR, Pienta KJ, Targeting Tyro3. Axl and MerTK (TAM receptors): implications for macrophages in the tumor microenvironment. Mol Cancer 18:94, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wan E, Yeap XY, Dehn S, Terry R, Novak M, Zhang S, Iwata S, Han X, Homma S, Drosatos K, et al. : Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res 113:1004–1012, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Suresh Babu S, Thandavarayan RA, Joladarashi D, Jeyabal P, Krishnamurthy S, Bhimaraj A, Youker KA, Krishnamurthy P: MicroRNA-126 overexpression rescues diabetes-induced impairment in efferocytosis of apoptotic cardiomyocytes. Sci Rep 6:36207, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dolmatova E, Spagnol G, Boassa D, Baum JR, Keith K, Ambrosi C, Kontaridis MI, Sorgen PL, Sosinsky GE, Duffy HS: Cardiomyocyte ATP release through pannexin 1 aids in early fibroblast activation. Am J Physiol Heart Circ Physiol 303:H1208–H1218, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.King KR, Aguirre AD, Ye YX, Sun Y, Roh JD, Ng RP Jr, Kohler RH, Arlauckas SP, Iwamoto Y, Savol A, et al. : IRF3 and type I interferons fuel a fatal response to myocardial infarction. Nat Med 23:1481–1487, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Poon IK, Lucas CD, Rossi AG, Ravichandran KS: Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol 14:166–180, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LA, Perretti M, Rossi AG, Wallace JL: Resolution of inflammation: state of the art, definitions and terms. FASEB J 21:325–332, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, Weber C, Soehnlein O, Steffens S: Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J 38:187–197, 2017. [DOI] [PubMed] [Google Scholar]
- 114.Greenlee-Wacker MC: Clearance of apoptotic neutrophils and resolution of inflammation. Immunol Rev 273:357–370, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Werner F, Jain MK, Feinberg MW, Sibinga NE, Pellacani A, Wiesel P, Chin MT, Topper JN, Perrella MA, Lee ME: Transforming growth factor-beta 1 inhibition of macrophage activation is mediated via Smad3. J Biol Chem 275:36653–36658, 2000. [DOI] [PubMed] [Google Scholar]
- 116.Sanjabi S, Oh SA, Li MO: Regulation of the immune response by TGF-β: from conception to autoimmunity and infection. Cold Spring Harb Perspect Biol 9:a022236, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen B, Huang S, Su Y, Wu YJ, Hanna A, Brickshawana A, Graff J, Frangogiannis NG: Macrophage Smad3 protects the infarcted heart, stimulating phagocytosis and regulating inflammation. Circ Res 125:55–70, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Heidt T, Courties G, Dutta P, Sager HB, Sebas M, Iwamoto Y, Sun Y, Da Silva N, Panizzi P, van der Laan AM, et al. : Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res 115:284–295, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peet C, Ivetic A, Bromage DI, Shah AM: Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc Res 116:1101–1112, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hilgendorf I, Gerhardt LM, Tan TC, Winter C, Holderried TA, Chousterman BG, Iwamoto Y, Liao R, Zirlik A, Scherer-Crosbie M, et al. : Ly-6Chigh monocytes depend on Nr4al to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res 114:1611–1622, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ: The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204:3037–3047, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marinković G, Koenis D, de Camp L, Jablonowski R, Graber N, de Waard V, de Vries CJ, Goncalves I, Nilsson J, Jovinge S, et al. : S100A9 links inflammation and repair in myocardial infarction. Circ Res; 2020. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 123.Dehn S, Thorp EB: Myeloid receptor CD36 is required for early phagocytosis of myocardial infarcts and induction of Nr4al-dependent mechanisms of cardiac repair. FASEB J 32:254–264, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC: The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat Immunol 12:778–785, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tacke R, Hilgendorf I, Garner H, Waterborg C, Park K, Nowyhed H, Hanna RN, Wu R, Swirski FK, Geissmann F, et al. : The transcription factor NR4A1 is essential for the development of a novel macrophage subset in the thymus. Sci Rep 5:10055, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Koenis DS, Medzikovic L, van Loenen PB, van Weeghel M, Huveneers S, Vos M, Evers-van Gogh IJ, Van den Bossche J, Speijer D, Kim Y, et al. : Nuclear receptor Nur77 limits the macrophage inflammatory response through transcriptional reprogramming of mitochondrial metabolism. Cell Rep 24.2127–2140.e7,2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C: Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116:829–840, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Corliss BA, Azimi MS, Munson JM, Peirce SM, Murfee WL: Macrophages: an inflammatory link between angiogenesis and lymphangiogenesis. Microcirculation 23:95–121, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pinto AR, Paolicelli R, Salimova E, Gospocic J, Slonimsky E, Bilbao-Cortes D, Godwin JW, Rosenthal NA: An abundant tissue macrophage population in the adult murine heart with a distinct alternatively activated macrophage profile. PLoS One 7:e36814, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Frangogiannis NG: The role of transforming growth factor (TGF)-β in the infarcted myocardium. J Thorac Dis 9:S52–S63, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao T, Zhao W, Chen Y, Ahokas RA, Sun Y: Vascular endothelial growth factor (VEGF)-A: role on cardiac angiogenesis following myocardial infarction. Microvasc Res 80:188–194, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cai B, Thorp EB, Doran AC, Subramanian M, Sansbury BE, Lin CS, Spite M, Fredman G, Tabas I: MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc Natl Acad Sci U S A 113:6526–6531, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Howangyin KY, Zlatanova I, Pinto C, Ngkelo A, Cochain C, Rouanet M, Vilar J, Lemitre M, Stockmann C, Fleischmann BK, et al. : Myeloid-epithelialreproductive receptor tyrosine kinase and milk fat globule epidermal growth factor 8 coordinately improve remodeling after myocardial infarction via local delivery of vascular endothelial growth factor. Circulation 133:826–839,2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gong D, Shi W, Yi SJ, Chen H, Groffen J, Heisterkamp N: TGFβ signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol 13:31, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Avraham-Davidi I, Yona S, Grunewald M, Landsman L, Cochain C, Silvestre JS, Mizrahi H, Faroja M, Strauss-Ayali D, Mack M, et al. : On-site education of VEGF-recruited monocytes improves their performance as angiogenic and arteriogenic accessory cells. J Exp Med 210:2611–2625, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.O’Rourke SA, Dunne A, Monaghan MG: The role of macrophages in the infarcted myocardium: orchestrators of ECM remodeling. Front Cardiovasc Med 6:101, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hu Y, Zhang H, Lu Y, Bai H, Xu Y, Zhu X, Zhou R, Ben J, Xu Y, Chen Q: Class A scavenger receptor attenuates myocardial infarction-induced cardiomyocyte necrosis through suppressing Ml macrophage subset polarization. Basic Res Cardiol 106:1311–1328, 2011. [DOI] [PubMed] [Google Scholar]
- 138.Peiser L, Gough PJ, Kodama T, Gordon S: Macrophage class A scavenger receptor-mediated phagocytosis of Escherichia coli: role of cell heterogeneity, microbial strain, and culture conditions in vitro. Infect Immun 68:1953–1963, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.PrabhuDas MR, Baldwin CL, Bollyky PL, Bowdish DME, Drickamer K, Febbraio M, Herz J, Kobzik L, Krieger M, Loike J, et al. : A consensus definitive classification of scavenger receptors and their roles in health and disease. J Immunol 198:3775–3789, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Platt N, Gordon S: Is the class A macrophage scavenger receptor (SR-A) multifunctional? – The mouse’s tale. J Clin Invest 108:649–654, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tsujita K, Kaikita K, Hayasaki T, Honda T, Kobayashi H, Sakashita N, Suzuki H, Kodama T, Ogawa H, Takeya M: Targeted deletion of class A macrophage scavenger receptor increases the risk of cardiac rupture after experimental myocardial infarction. Circulation 115:1904–1911, 2007. [DOI] [PubMed] [Google Scholar]
- 142.Iyer RP, Jung M, Lindsey ML: MMP-9 signaling in the left ventricle following myocardial infarction. Am J Physiol Heart Circ Physiol 311:H190–H198, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, Dyspersin GD, Cleutjens JP, Shipley M, Angellilo A, et al. : Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med 5:1135–1142, 1999. [DOI] [PubMed] [Google Scholar]
- 144.DeLeon-Pennell KY, Tian Y, Zhang B, Cates CA, Iyer RP, Cannon P, Shah P, Aiyetan P, Halade GV, Ma Y, et al. : CD36 is a matrix metalloproteinase-9 substrate that stimulates neutrophil apoptosis and removal during cardiac remodeling. Circ Cardiovasc Genet 9:14–25, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Eltzschig HK, Eckle T: Ischemia and reperfusion—from mechanism to translation. Nat Med 17:1391–1401, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ren D, Wang X, Ha T, Liu L, Kalbfleisch J, Gao X, Williams D, Li C: SR-A deficiency reduces myocardial ischemia/reperfusion injury; involvement of increased microRNA-125b expression in macrophages. Biochim Biophys Acta 1832:336–346, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vagnozzi RJ, Maillet M, Sargent MA, Khalil H, Johansen AKZ, Schwanekamp JA, York AJ, Huang V, Nahrendorf M, Sadayappan S, et al. : An acute immune response underlies the benefit of cardiac stem cell therapy. Nature 577:405–409, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shiraishi M, Shintani Y, Shintani Y, Ishida H, Saba R, Yamaguchi A, Adachi H, Yashiro K, Suzuki K: Alternatively activated macrophages determine repair of the infarcted adult murine heart. J Clin Invest 126:2151–2166, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Madsen DH, Leonard D, Masedunskas A, Moyer A, Jurgensen HJ, Peters DE, Amornphimoltham P, Selvaraj A, Yamada SS, Brenner DA, et al. : M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J Cell Biol 202:951–966, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hou X, Chen G, Bracamonte-Baran W, Choi HS, Diny NL, Sung J, Hughes D, Won T, Wood MK, Talor MV, et al. : The cardiac microenvironment instructs divergent monocyte fates and functions in myocarditis. Cell Rep 28. 172–189. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wu L, Ong S, Talor MV, Barin JG, Baldeviano GC, Kass DA, Bedja D, Zhang H, Sheikh A, Margolick JB, et al. : Cardiac fibroblasts mediate IL-17A-driven inflammatory dilated cardiomyopathy. J Exp Med 211:1449–1464, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Walker GA, Masters KS, Shah DN, Anseth KS, Leinwand LA: Valvular myofibroblast activation by transforming growth factor-beta: implications for pathological extracellular matrix remodeling in heart valve disease. Circ Res 95:253–260, 2004. [DOI] [PubMed] [Google Scholar]
- 153.Swinnen M, Vanhoutte D, Van Almen GC, Hamdani N, Schellings MW, D’hooge J, Van der Velden J, Weaver MS, Sage EH, Bornstein P, et al. : Absence of thrombospondin-2 causes age-related dilated cardiomyopathy. Circulation 120:1585–1597, 2009. [DOI] [PubMed] [Google Scholar]
- 154.Izmirly PM, Saxena A, Kim MY, Wang D, Sahl SK, Llanos C, Friedman D, Buyon JP: Maternal and fetal factors associated with mortality and morbidity in a multi-racial/ethnic registry of anti-SSA/Ro-associated cardiac neonatal lupus. Circulation 124:1927–1935, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang S, Yeap XY, DeBerge M, Naresh NK, Wang K, Jiang Z, Wilcox JE, White SM, Morrow JP, Burridge PW, et al. : Acute CD47 blockade during ischemic myocardial reperfusion enhances phagocytosis-associated cardiac repair. JACC Basic Transl Sci 2:386–397, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sharifi-Sanjani M, Shoushtari AH, Quiroz M, Baust J, Sestito SF, Mosher M, Ross M, McTiernan CF, St Croix CM, Bilonick RA, et al. : Cardiac CD47 drives left ventricular heart failure through Ca2+-CaMKII-regulated induction of HDAC3. J Am Heart Assoc 3:e000670, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, et al. : Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 380:752–762, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. : Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 377:1119–1131, 2017. [DOI] [PubMed] [Google Scholar]
- 159.verett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ, Ridker PM: Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation 139:1289–1299, 2019. [DOI] [PubMed] [Google Scholar]
- 160.Kelley JL, Ozment TR, Li C, Schweitzer JB, Williams DL: Scavenger receptor-A (CD204): a two-edged sword in health and disease. Crit Rev Immunol 34:241–261, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]