Abstract
There is much evidence pointing out eye movement alterations in several neurological diseases. To the best of our knowledge, this is the first video-oculography study describing potential alterations of eye movements in the post-COVID-19 condition. Visually guided saccades, memory-guided saccades, and antisaccades in horizontal axis were measured. In all visual tests, the stimulus was deployed with a gap condition. The duration of the test was between 5 and 7 min per participant. A group of patients with the post-COVID-19 condition was included in this study. Values were compared with a group () of healthy volunteers whom the SARS-CoV-2 virus had not infected. Features such as centripetal and centrifugal latencies, success rates in memory saccades, antisaccades, and blinks were computed. We found that patients with the post-COVID-19 condition had eye movement alterations mainly in centripetal latency in visually guided saccades, the success rate in memory-guided saccade test, latency in antisaccades, and its standard deviation, which suggests the involvement of frontoparietal networks. Further work is required to understand these eye movements’ alterations and their functional consequences.
Keywords: pathophysiology, eye movement, wearable gaze-tracker, post-COVID-19 condition, saccadic movement
1. Introduction
The measurement of eye movement and its alteration arises as a powerful marker to diagnose brain functionality. Several studies support this fact. For instance, in [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17] provide scientific pieces of evidence related to particular alterations of eye movement in multiple neurological disorders, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), and autism, among others. For example, patients with AD not only have higher latency and latency variability regardless of the tasks, but also show more incorrect antisaccades and take more time to correct them, which has been suggested as an early marker of AD [3,4,5,6,7]. In PD and some other parkinsonian syndromes, patients may present eye movement alterations, such as hypometria, abnormally fragmented saccades (multistep or staircase saccades), and considerable difficulty in inhibiting the saccade movement reflex during the antisaccade test. Furthermore, in patients with moderate or advanced PD, the latency in the visually guided saccades are higher compared with the controls [1,9].
In saccadic movements, several processes take place in the brain. For example, during the latency period, a shift of visual attention to the new target, a disengagement of oculomotor fixation, and a computation of the metrics of the movement are performed by the subject. These processes involve the activation of a large circuit that involves different cortical areas, including the parietal and the frontal lobes. Hence, the latency of eye movement is a cognitive–physiological parameter [11,15,16].
Coronavirus disease 2019 (COVID-19) primarily affects the respiratory system causing bilateral pneumonia, but it is increasingly being recognized as a systemic disease, with neurological manifestations reported even in patients with mild respiratory symptoms [18,19,20,21,22,23,24,25]. In fact, some patients with COVID-19 have clinical pictures similar to central nervous system infections, such as headache, epilepsy, and disturbed consciousness [24].
Neurotropism of SARS-CoV-2 infection has been established beyond doubt [18,19,20,21,22,23,24,25]. In this sense, many clinicians are expecting to see cases of post-COVID-19 neurological sequelae, mainly movement disorders [22] and other neurodegenerative diseases [26] in the upcoming decade.
There is little information about cognitive impairment in COVID-19 survivors. In a retrospective study in a large academic medical center in Chicago, Illinois, that included 50 hospitalized patients, 12 (24%) had cognitive abnormalities, particularly short-term memory impairment [27]. In a British cross-specialty surveillance study of acute neurological and psychiatric complications of COVID-19 that included 23 patients with neuropsychiatric disorders, six of them (26%) had a neurocognitive (dementia-like) syndrome [28]. There is also preliminary evidence of cognitive impairment after hospital discharge characterized by inattention, disorientation, and poorly organized movements in response to commands [29]. In a case series of four severe COVID-19 patients who required intensive care unit admission, cognitive impairment, identified as memory deficit and frontal syndrome, was detected after discharge [30].
We are now seeing that many COVID-19 survivors might have sustained postinfection sequelae, including fatigue, shortness of breath, and cognitive dysfunction, among others, which generally have an impact on everyday functioning [31]. These sequelae occur in patients with a history of probable or confirmed SARS-CoV-2 infection, usually three months from the onset, with symptoms that last for at least two months and cannot be explained by an alternative diagnosis. Known by a variety of names, including long COVID or long-haul COVID, are listed in the ICD-10 classification as post-COVID-19 condition [31].
Our aim is to study the potential eye movement alterations in post-COVID-19 patients. This assessment has the property of being non-invasive and cost-effective. Moreover, it is widely used to assess cognitive function in some neurological diseases, as previously mentioned. In this proof-of-concept, binocular eye movement was recorded using video-oculography. Images were processed under Matlab environments, and the most relevant features were computed and analyzed. To record the eye movement, we used a commercial and wearable gaze-tracker while a conventional chin rest immobilized the head of the participants. The visual stimulus was deployed in a conventional screen placed at 60 cm from the patient (the typical setup in all eye movements experiments) and its battery was programmed under a Python environment on an open-source platform called PsychoPy.
The ethical committees of the University Hospital 12 de Octubre, Madrid, Spain, approved this research.
The present article is organized as follows: Section 2 describes the Materials and Methods used in this proof-of-concept. Additionally, the full description of the eye movement tests, hardware, software, demographics, and clinical data of patients are deeply described in that section. Results are presented in Section 3, while the main findings are discussed in Section 4. Finally, conclusions and further clinical studies are summarized in Section 5.
2. Materials and Methods
A saccadic movement, or saccades, is defined as a rapid jerk-like movement of the eyes that direct the gaze to a new location and redeploy the region of high visual acuity centered on the fovea [32]. Saccades are regarded as voluntary movements, but are generally produced with highly automated routines. For further information related to the neurological aspects of eye movements, the reader is referred to [33].
2.1. Hardware and Software Description
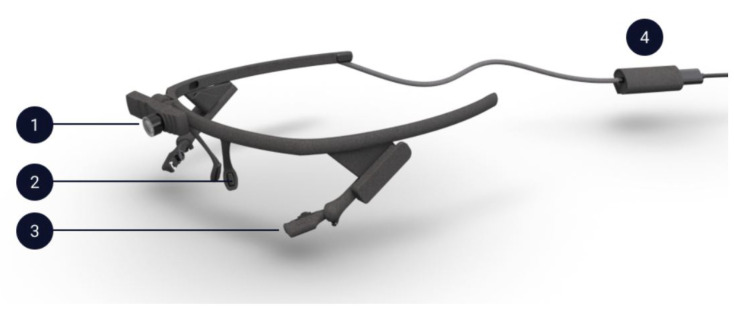
A commercial, binocular and wearable gaze tracker was used to record the eye movements. A picture of this gaze tracker model is shown in Figure 1 [34].
Figure 1.
Pupil Core headset: (1) World Camera, (2) Nose Support, (3) Eye Cameras and (4) USB-C connector clip.
This whole system weighs around 22.75 g, and its dimensions are 160 mm × 51 mm × 175 mm. While the world camera is used to capture the image of the whole visual field of the user, each eye camera records the pupil position using infrared light. In this setup, the world camera operates at 30 Hz and each eye camera at 200 Hz with a resolution of 1080 × 1080 px and 192 × 192 px, respectively. According to user manual data, the gaze accuracy is 0.60º with a precision of 0.02º [35]. Moreover, the technical parameters reported by the manufactured turns it suitable for this proof-of-concept.
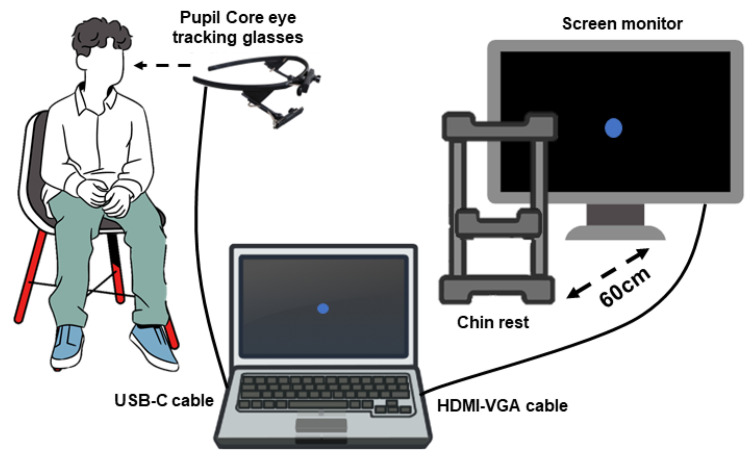
The subject wearing the gaze tracker was seated in front of the monitor with his/her head in the chin rest. We used a chin rest to keep the head still to avoid disturbance in the measurement of eye movements. The chin rest was placed at 60 cm from the monitor used to deploy the visual stimulus. The center of the monitor and the user’s line of sight were coincident as much as possible. The monitor was connected by an HDMI/VGA cable to a computer (ASUS VivoBook laptop CORE i7 8th Gen, in this specific case), while the Pupil Core eye-tracking glasses were also connected to the same laptop via USB-C cable. Figure 2 shows a scheme of the system setup. Notice that corrective glasses and make-up have to be removed.
Figure 2.
Scheme of the eye movements tests’ setup.
The video-oculography system was calibrated before the recording using the calibration software provided by the manufacturer. It consisted of the onset of the stimulus in five different positions on the screen: the center and the four corners. The targets remained in each position for a few seconds while the pupil was automatically detected and that particular position of the gaze recorded.
In order to generate the battery of visual stimulus, it was used an open-source software called PsychoPy [36,37]. It is a free cross-platform package that allows running a wide range of experiments in the behavioral sciences (neuroscience, psychology, psychophysics, and linguistics, among others). It is a community project, so users have all the source code available to develop a new application. Moreover, it has a flexible and intuitive Builder Interface to develop a new experiment through a Python code or using the resources available in the application such as texts, icons, and figures, among others.
It is worth emphasizing that Pupil software and PsychoPy software are manually synchronized, then the user is encouraged to stay quiet and not move the head after the calibration. Room lights were turned off to enhance the quality of the captured image by the gaze tracker. The procedure is outlined in Figure 3.
Figure 3.
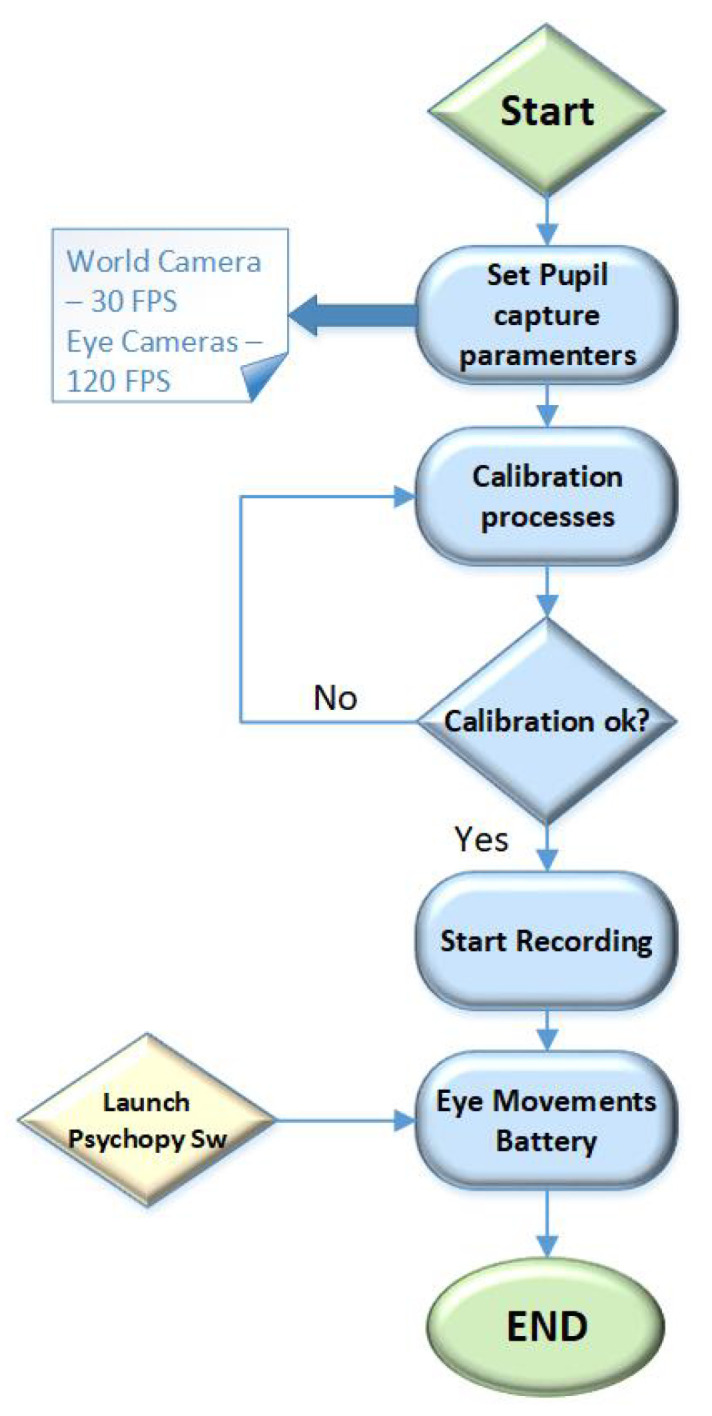
Eye movements test procedure.
2.2. Description of the Eye Movement Battery
Based on previous studies [3,38,39,40,41,42] and as well as in authors expertise [2,16,43,44,45,46], in this proof of concept, it was decided to test the horizontal axis to validate the potential of the eye movement as a biomarker for the brain functionality in the post-COVID-19 condition.
In order to assess saccadic movements, there are three methods: the gap where the fixation point disappears prior to the onset of the visual stimulus; the shift where the offset of the fixation point and the onset of the visual stimulus happen simultaneously; and the overlap paradigms where the fixation point and the visual stimulus are overlapped in time before the offset of the former [47,48,49,50].
Saccadic latency is defined as the time (in milliseconds) from target appearance to saccade initiation of correct trials. Latency value is task dependant. In fact, there are several studies reporting these differences [5,17,51,52,53,54,55,56,57,58,59,60]. Perhaps, the most significant factor in reducing the reaction time, in terms of both the magnitude and robustness of the reduction, occurs in a phenomenon known as the gap. In a typical gap effect study, participants are asked to fixate on a centrally located fixation point and then make a saccade, as quickly as possible, to a suddenly appearing peripheral target. Several pieces of research justify the use of “gap” in attention and memory assessments such as.
This study implemented three visual tests under gap conditions: visually guided saccades, memory-guided saccades, and antisaccades. A calibration process was carried out before each test to ensure the accuracy of the measurement. As a stimulus, a blue circle with a diameter of 3 mm was selected, and the screen background was configured in dark black color. The stimulus location was , and . In all tests, the position of the stimulus (left or right, as well as the target amplitude) was randomized, and the gap duration was three seconds. In the following section, details related to each paradigm test are mentioned.
2.2.1. Horizontal Visually Guided Saccade Test
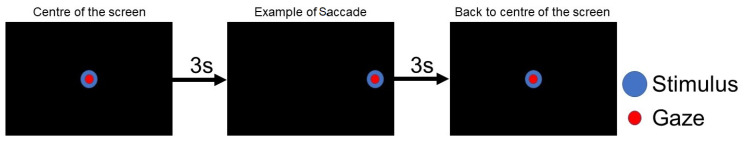
As shown in Figure 4, the visual stimulus appears in the center of the screen and jumps, randomly, towards a new position and, after three seconds, returns to the center. The whole duration of the test was one minute, and this process was repeated ten times. In this test, variables like latency towards the stimulus, latency back to the center of the screen, number of blinks, and performance were measured.
Figure 4.
Horizontal visually guided saccade test.
2.2.2. Horizontal Memory-Guided Saccade Test
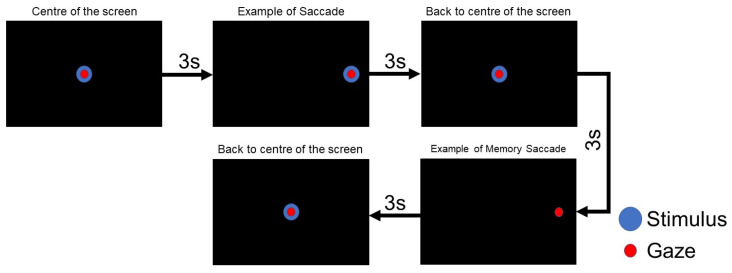
In this test, the working memory [61,62] was assessed. We instructed to the subject to make a prosaccade during the first stimulus’s onset and then to remember this position in order to do a memory saccade in the dark period. Figure 5 summarizes this procedure: the visual stimulus appears in the center of the screen, after 3 s, the stimulus jumps randomly to right or left, with unpredictable eccentricity, , and and returns to the screen’s center. After that, the participant must remember the last position of the visual stimulus and generate a voluntary eye movement towards it, but in the absence of a stimulus. The whole duration of the test is two minutes, and variables such as blinks and success rate in the memory saccade are computed.
Figure 5.
Horizontal memory guided saccade test.
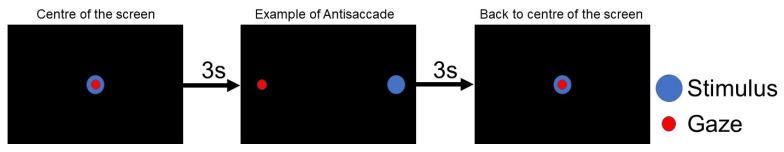
2.2.3. Horizontal Visually Antisaccade Test
Similar to the horizontal visually guided saccade test, in the antisaccade test, the stimulus appears first in the center and then in random positions for three seconds in both cases. However, the participant is encouraged to look at the opposite position of the visual stimulus. This process is repeated ten times, and the whole duration of the test is one minute. In Figure 6, a scheme of antisaccade test is presented. The performance of the antisaccade test requires at least two subprocesses: the ability to suppress a reflexive saccade towards the visual stimulus and the ability to generate a voluntary saccade in the opposite direction to a location without any stimulus. Blinks, success in the antisaccades, the latency of the antisaccades and reflexive antisaccades were calculated.
Figure 6.
Antisaccade test scheme.
2.2.4. Eye Movement Features Characterization
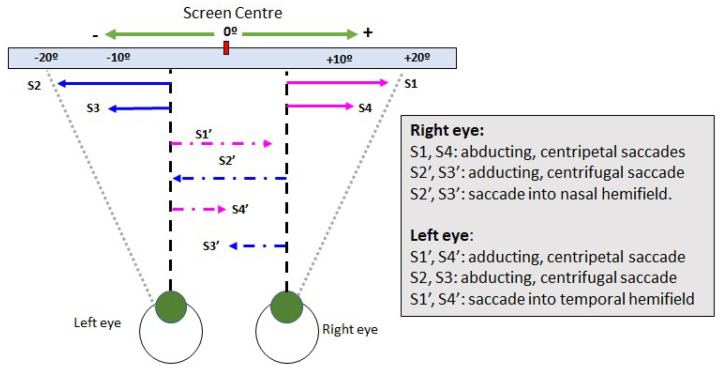
This subsection defines the features computed in eye movements tests described in the previous subsection. After recording, each test is processed using the gaze-tracker software that provides binocular gaze positions in both axes, x, and y. Figure 7 shows a scheme with the saccadic movements for each eye. Here, the movement is classified according to the movement itself (adducting or abducting), the visual pathway (temporal or nasal), and the primary position of the eyeball (centrifugal and centripetal). Centripetal saccades are made from the periphery of the orbit to its center (the primary position of the eyeball), while centrifugal saccades are made from the center to the periphery. These saccadic movements involve the adduction and the abduction of the eyeball.
Figure 7.
Binocular saccadic movements scheme.
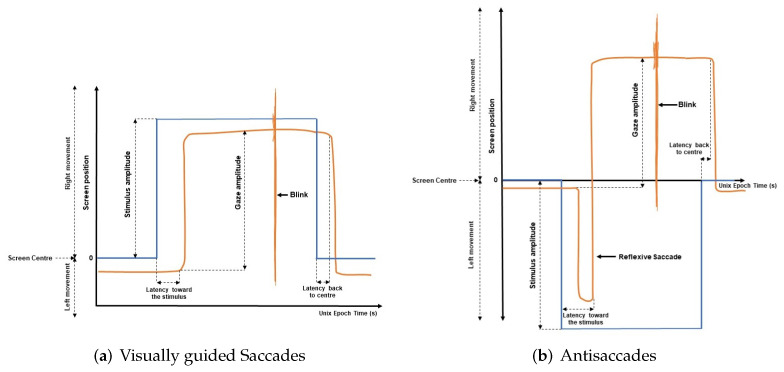
Figure 8 shows the features considered in this research. Blinks are automatically detected and exported by the gaze tracker software.
Figure 8.
Gap paradigm. Features computed in the eye movement tests.
Centrifugal latency, [ms], is defined by the time elapsed between the onset of the periphery stimulus, , and the time from the eye moves in response to this stimulus . It is computed by (1);
| (1) |
Centripetal Latency, [ms], is defined by the time elapsed between the onset of the fixation point in the center of the screen, , and the time from the eye moves in response to it, . In this case, it is computed by (2);
| (2) |
In [44] was demonstrated the linear relation between age and the and for healthy volunteers, and a model was provided for computing the latency under gap paradigm. That research used the centrifugal and centripetal latencies model to compare the measured values to ensure the measured value’s reliability. In (3) and (4), the linear dependency between latency and age in healthy brain aging was demonstrated.
| (3) |
| (4) |
According to [16], Reflexive Saccade is defined by an eye movement in the same direction of the stimulus, in the antisaccade test (see Figure 8b). The Success Rate in antisaccade test or in memory guided test is defined by the correct movement according to the test (opposite side in antisaccade and remember position in memory test, respectively).
When the centripetal latency, , is between 70 ms and 130 ms, the eye movement is usually referred to as express saccade [63] and is driven by the subcortical retinotectal pathways [64]. In contrast, the “normal” saccades are driven by the thalamocortical pathway, which projects to the parietal eye field and then to the superior colliculus [65], so express saccade is not included in mean values.
This study did not include measurements of dysmetrias and related variables and asymmetries in the movement (differences between eyes).
2.3. Healthy Controls and Patients with the Post-COVID-19 Condition
A group of ten patients with the post-COVID-19 condition was consecutively selected from the Patient Affected by COVID-19 Disease Association (AMACOVID), Madrid. None reported having a neurological disease previous to COVID-19. One of the subjects was excluded after the data analysis due to noise in the measurement; therefore, nine participants (three men and six women) were included in the analyses. The patients with the COVID-19 condition had a mean (±standard deviation) age of 49.56 ± 9.14 years, weight of 74.33 ± 19.29 kg, and height of 164.0 ± 7.27 cm. On the other hand, a group of healthy volunteers matched by age was recruited as a control group. These subjects had not been diagnosed with COVID-19 disease nor had previous neurological diseases [44]. See Table 1 for details.
Table 1.
Demographic and clinical characteristics of healthy controls.
| ID | Age (Range) | Sex | Cardiovascular Diseases | Diabetes Mellitus |
|---|---|---|---|---|
| H1 | 51–60 | Female | Arterial hypertension | No |
| H2 | <19 | Male | Arrhythmia | No |
| H3 | <19 | Male | None | No |
| H4 | 20–40 | Male | None | No |
| H5 | 20–40 | Male | None | No |
| H6 | 20–40 | Male | None | No |
| H7 | 20–40 | Female | None | No |
| H8 | 20–40 | Male | None | No |
| H9 | 41–50 | Male | None | No |
Table 2 shows the most relevant demographic and clinical characteristics of post-COVID-19 patients.
Table 2.
Demographic, clinical characteristics and medical history of patients before COVID-19 diagnosis.
| Patient | Age | Sex | Laboral Activity | Cardiovascular Diseases | Diabetes Mellitus |
|---|---|---|---|---|---|
| VAMA01 | 59 | Female | Association coordinator | None | Yes |
| VAMA02 | 54 | Male | Commercial tasks | Supraventricular arrythmia (14y) | No |
| VAMA03 | 28 | Female | Housewife | None | No |
| VAMA04 | 44 | Female | Housewife | None | No |
| VAMA05 | 60 | Male | Caretaker and maintenance in a school | None | No |
| VAMA06 | 49 | Female | Cleaner | None | No |
| VAMA08 | 46 | Female | Call center | None | No |
| VAMA09 | 54 | Male | Worker | None | No |
| VAMA10 | 52 | Female | Call center | None | No |
Table 3 summarizes the clinical picture suffered by the patients upon the diagnosis, including the diagnosis date. COVID-19 diagnosis was confirmed by SARS-CoV-2 reverse transcription-polymerase chain reaction of a nasopharyngeal swab. Three patients were infected during the first wave (February to 1 June 2020), four were infected during the second wave (2 June 2020–9 December 2020), and the remaining two during the third wave (10 December 2020–15 March 2021). The most common clinical picture was febrile illness followed by cough and pneumonia. Patients’ other symptoms were hair and skin problems, headache, anorexia, and diarrhea, among others.
Table 3.
Clinical picture upon diagnosis.
| Patient | Diagnosis Date (mm/dd/yyyy) |
Diarrhea | Pneumonia | Fever | Cough | Headache | Gastrointestinal Symptoms |
|---|---|---|---|---|---|---|---|
| VAMA01 | 04/04/2020 | X | X | X | - | - | - |
| VAMA02 | 03/03/2020 | - | X | X | X | - | - |
| VAMA03 | 08/28/2020 | X | - | X | - | X | X |
| VAMA04 | 12/17/2020 | - | X | X | X | X | Anorexia |
| VAMA05 | 09/03/2020 | - | X (bilateral) | X | X | - | Anorexia |
| VAMA06 | 12/29/2020 | - | - | X | X | - | - |
| VAMA08 | 12/29/2020 | - | - | X | - | X | - |
| VAMA09 | 12/02/2020 | - | Mild | Low-grade | - | - | - |
| VAMA10 | 03/15/2020 | X | - | - | X | X | X |
Table 4 summarizes the symptoms suffered by patients after COVID-19. The majority of them present complaints of fatigue or memory disturbance. Other symptoms reported were anosmia and shortness of breath. Patients VAMA02 and VAMA03 mentioned abnormal heart rhythms, such as arrhythmias and tachycardia, respectively. On the other hand, VAMA03 had edemas of the lower limbs and VAMA10 skin rash; hair loss was reported in both patients. This last patient also complained of hand pain and VAMA06 that had limb weakness. In addition, VAMA03 had speech problems and hand pain. VAMA06 had limb weakness as well.
Table 4.
Post-COVID-19 symptoms.
| Patient | Anosmia | Memory Complaints | Fatigue | Shortness of Breath |
|---|---|---|---|---|
| VAMA01 | X | X | X | - |
| VAMA02 | - | - | X | X |
| VAMA03 | - | X | - | X |
| VAMA04 | X | X | X | - |
| VAMA05 | - | - | X | X (on walking) |
| VAMA06 | - | X | X | - |
| VAMA08 | X | X | X | - |
| VAMA09 | - | X | X | X |
| VAMA10 | - | X | X | X (feeling of chest pressure) |
3. Results
3.1. Validation of the Measurement of the Group of Healthy Volunteers
Table 5, Table 6 and Table 7 present the computed eye movement’s features described in previous section. Table 5 shows the minimum, maximum, and mean value of the latency, as well as the value computed by the model given by Equations (3) and (4). By computing the absolute error in each measurement, we found that this error ranged between and ms; hence, the values measured with the portable gaze tracker had the accuracy needed to compute latency.
Table 5.
Visually guided saccade test: latencies values for the control group.
| ID | Blinks |
[s] – |
± SD | ± SD | ||
|---|---|---|---|---|---|---|
| H1 | 0 | 0.198–0.272 | 0.223 ± 0.02 | 0.219 | 0.213 ± 0.02 | 0.214 |
| H2 | 3 | 0.132–0.451 | 0.212 ± 0.09 | 0.197 | 0.148 ± 0.02 | 0.178 |
| H3 | 0 | 0.151–0.383 | 0.212 ± 0.06 | 0.197 | 0.202 ± 0.06 | 0.178 |
| H4 | 4 | 0.156–0.280 | 0.198 ± 0.03 | 0.200 | 0.216 ± 0.03 | 0.183 |
| H5 | 0 | 0.154–0.267 | 0.192 ± 0.03 | 0.202 | 0.189 ± 0.03 | 0.184 |
| H6 | 2 | 0.132–0.239 | 0.181 ± 0.03 | 0.199 | 0.162 ± 0.05 | 0.180 |
| H7 | 8 | 0.151–0.269 | 0.180 ± 0.03 | 0.201 | 0.203 ± 0.07 | 0.185 |
| H8 | 0 | 0.137–0.233 | 0.176 ± 0.03 | 0.203 | 0.172 ± 0.02 | 0.188 |
| H9 | 3 | 0.214–0.338 | 0.297 ± 0.06 | 0.211 | 0.208 ± 0.02 | 0.200 |
| Mean Values | 2.2 | 0.158–0.303 | 0.209 ± 0.04 | 0.203 | 0.190 ± 0.03 | 0.187 |
Table 6.
Memory-guided saccade test: success rate in memory saccade for healthy control group.
| ID | Blinks | Success Rate |
|---|---|---|
| H1 | 3 | 100% |
| H2 | 12 | 100% |
| H3 | 0 | 100% |
| H4 | 2 | 100% |
| H5 | 0 | 90% |
| H6 | 2 | 100% |
| H7 | 21 | 90% |
| H8 | 4 | 100% |
| H9 | 3 | 100% |
| Mean Values | 5.2 | 97.77% |
Table 7.
Antisaccadic test: main features for the healthy control group.
| ID | Blinks | Correct | Reflexive | Success Rate | Latency [s] Min–Max |
Mean ± SD |
|---|---|---|---|---|---|---|
| H1 | 2 | 100% | 37.5% | 62.5% | 0.160–0.659 | 0.316 ± 0.135 |
| H2 | 8 | 100% | 50% | 50% | 0.197–0.511 | 0.330 ± 0.092 |
| H3 | 0 | 100% | 60% | 40% | 0.219–0.612 | 0.336 ± 0.103 |
| H4 | 0 | 100% | 60% | 40% | 0.210–0.463 | 0.317 ± 0.078 |
| H5 | 1 | 80% | 40% | 40% | 0.216–0.679 | 0.396 ± 0.135 |
| H6 | 3 | 100% | 0% | 100% | 0.162–0.449 | 0.232 ± 0.079 |
| H7 | 0 | 70% | 40% | 30% | 0.122–0.672 | 0.368 ± 0.140 |
| H8 | 0 | 100% | 60% | 60% | 0.180–0.469 | 0.273 ± 0.092 |
| H9 | 1 | 100% | 50% | 50% | 0.197–0.398 | 0.315 ± 0.044 |
| Mean Values | 1.6 | 94.4% | 44.2% | 52.5% | 0.184–0.545 | 0.320 ± 0.099 |
Average antisaccade error rates in healthy humans vary considerably across studies and laboratories, with some studies reporting rates as low as 5% and others as high as 25% [66,67]. Some studies [68,69] using large samples suggest an error rate of around 20% is typical. Error rates are not constant across the lifespan, being highest during childhood, reaching the lower rates during early adulthood, and then increasing very slowly with advancing age until around 55–60, when the rate of increase appears to accelerate [44].
The latency values depend on the target location tested as well as the method to assess it (gap, step or overlap) [70]. Several studies report the values of latency in visually guided saccade test with a large number of volunteers [71,72,73,74]. By comparing results given in Table 7 with those previously reported, we found similar latency values. According to memory-guided saccades and antisaccades tests, parameters measured from group G1 were in the range of those previous studies related to the success of the ocular tasks [44,75,76]. The main conclusion here is that the gaze-tracker is helpful for this proof of concept.
3.2. Post-COVID-19 Patients Measurement
By considering the eye movement battery described in the previous section, the main results are presented in Table 8, Table 9 and Table 10. According to this, and by comparing them with those previously studies [18,77,78], these patients had eye movement alterations.
Table 8.
Visually guided saccade test: latencies values in post-COVID-19 patients.
| Patient | Blinks |
[s] – |
± SD | ± SD |
|---|---|---|---|---|
| VAMA01 | 2 | 0.162–0.327 | 0.216 ± 0.04 | 0.200 ± 0.02 |
| VAMA02 | 0 | 0.168–0.741 | 0.264 ± 0.16 | 0.255 ± 0.07 |
| VAMA03 | 0 | 0.177–0.361 | 0.248 ± 0.05 | 0.223 ± 0.03 |
| VAMA04 | 9 | 0.182–0.342 | 0.233 ± 0.05 | 0.214 ± 0.05 |
| VAMA05 | 6 | 0.168–0.330 | 0.234 ± 0.05 | 0.212 ± 0.05 |
| VAMA06 | 54 | 0.170–0.274 | 0.210 ± 0.03 | 0.213 ± 0.01 |
| VAMA08 | 11 | 0.167–0.302 | 0.226 ± 0.04 | 0.214± 0.04 |
| VAMA09 | 22 | 0.164–0.255 | 0.216 ± 0.03 | 0.223 ± 0.03 |
| VAMA10 | 3 | 0.214–0.712 | 0.496 ± 0.16 | 0.433 ± 0.15 |
| Mean Values | 11.8 | 0.174–0.404 | 0.260 ± 0.07 | 0.243 ± 0.05 |
Table 9.
Memory-guided saccade test: success rate in post-COVID-19 patients.
| Patient | Blinks | Success Rate |
|---|---|---|
| VAMA01 | 18 | 100% |
| VAMA02 | 0 | 80% |
| VAMA03 | 2 | 100% |
| VAMA04 | 18 | 100% |
| VAMA05 | 22 | 40% |
| VAMA06 | 53 | 0% |
| VAMA08 | 56 | 100% |
| VAMA09 | 20 | 100% |
| VAMA10 | 7 | 20% |
| Mean Values | 21.7 | 71.1% |
Table 10.
Antisaccadic test. Main features in post-COVID-19 patients.
| Patient | Blinks | Correct | Reflexive | Success Rate | Latency [sec] Min–Max |
Mean ± SD |
|---|---|---|---|---|---|---|
| VAMA01 | 9 | 100% | 10% | 90% | 0.227–0.630 | 0.438 ± 0.112 |
| VAMA02 | 1 | 60% | 100% | 0% | 0.177–0.737 | 0.472 ± 0.184 |
| VAMA03 | 0 | 100% | 90% | 10% | 0.272–0.934 | 0.417 ± 0.149 |
| VAMA04 | 5 | 100% | 60% | 40% | 0.240–0.831 | 0.432 ± 0.137 |
| VAMA05 | 4 | 100% | 40% | 60% | 0.219–0.860 | 0.333 ± 0.157 |
| VAMA06 | 34 | 70% | 40% | 30% | 0.227–1.499 | 0.461 ± 0.326 |
| VAMA08 | 26 | 90% | 30% | 60% | 0.257–0.574 | 0.358 ± 0.081 |
| VAMA09 | 27 | 90% | 70% | 20% | 0.246–0.888 | 0.468 ± 0.182 |
| VAMA10 | 7 | 80% | 60% | 20% | 0.275–0.948 | 0.499 ± 0.183 |
| Mean Values | 12.5 | 87.8% | 55.5% | 36.6% | 0.237–0.878 | 0.430 ± 0.167 |
Two patients had latencies out of the normal range for their age (VAMA02 and VAMA10). Considering the success rate in the memory-guided saccade test, three patients showed abnormal success rates (VAMA05, VAMA06, and VAMA10), less than 50%. In the antisaccade test, the total number of correct eye movements (opposite to the stimulus) were according to the normality; however, three patients had a poor performance in the inhibitory capabilities (VAMA02, VAMA06, and VAMA10). Furthermore, the latency of the antisaccades was proper of healthy volunteers in the range of 65–85 years old [44]. The number of blinks was also higher than healthy volunteers in all tests.
3.3. Statistical Analysis
Although the sample size in both groups was small, we checked the statistical significance of the features. Firstly, variables were classified as parametric or non-parametric. Most part of the values shown in Table 5, Table 6, Table 7, Table 8, Table 9 and Table 10 are expressed as the mean value and the standard deviation. Differences between groups were analyzed using t-test for parametric variables while Kruskal–Wallis test followed by Dunn’s test for non-parametric variables. This analysis was performed using MatLab.
There were significant differences among groups when p-value was less than 0.05. After this, significative variables were: centripetal latency in visually guided saccades (p-value < 0.05), the success rate in memory-guided saccade test had a p-value < 0.05, blinks in whole test (p-value < 0.02), latency in antisaccades (p-value < 0.001) and its standard deviation (p-value <0.001). Although the p-value for the centrifugal latency was not significant (>0.05), it could be due to the small sample size.
4. Discussion
In this study, we have reported the eye movement alterations in a group of post-COVID-19 patients. Despite the gaze tracker was not certified as a medical device, the computed values from the gaze position given by the gaze tracker were in agreement with the literature (see Section 3). Therefore, the gaze-tracker and the software developed to assess the main features are valid.
According to [70], using monocular recordings of eye movement in monocular viewing conditions or binocular recordings in binocular viewing conditions leads to asymmetries in the retina and the optic nerve, which affects saccadic eye movements, meanly in peak velocity than other parameters [79]. This study measured binocular eye movements and compared results with other studies done in binocular viewing conditions.
The values measured reveal that patients who have suffered COVID-19 have eye movement alterations of interest, as these alterations are similar to those reported for mild cognitive impairment or Alzheimer’s disease [3,4,5,6,7,9,11,12,15,16].
The dorsolateral prefrontal cortex is involved in the development of saccadic eye movements [80]. This structure has direct connections with the main cortical ocular motor areas, namely the frontal eye field and the supplementary eye field in the frontal lobe; several (associative, attentional, and motor) areas in the posterior parietal cortex, including the parietal eye field; the cingulate eye field in the anterior cingulate cortex; and the superior colliculus in the brainstem [80]. For the performance of memory-guided saccades, that is, involving short-term spatial memory, visually guided saccades, and antisaccades, the posterior parietal cortex, the dorsolateral prefrontal cortex, and the frontal eye field play significant roles [80,81,82,83]. We found that patients with the post-COVID-19 condition had eye movement alterations mainly in centripetal latency in visually guided saccades, the success rate in memory-guided saccade test, latency in antisaccades, and its standard deviation, which suggests the involvement of frontoparietal networks. Further work is required to understand these eye movements’ alterations and their functional consequences.
The main limitations of our study are the sample size and the age of volunteers. We, however, considered that the sample size was enough for a proof-of-concept study. Likewise, related to the second limitation, the age as well as the sex of the participants were not relevant to demonstrating that SARS-CoV-2 virus infection may affect eye movement performance.
5. Conclusions
This study aimed to evaluate eye movement alterations using wearable and a commercial gaze-tracker.
We used a chin rest to avoid the influence of head movements on eye movements. In addition, after each visual test, the system was re-calibrated. Furthermore, those ocular features highly dependent on the accuracy were not included, such as gain or dysmetria.
Related to the visual tests, we implemented them using a free software platform under Python. The gap setup was selected because it is widely used to assess memory conditions in other neurological diseases like mild cognitive impairment, AD, or PD, (see Section 1 and Section 2).
Our patients still report cognitive complaints that they did not have before COVID-19. Eye movement assessment objectively unveiled the existence of these symptoms. Ongoing, more extensive studies will better understand the impact of COVID-19 on the brain.
Acknowledgments
The authors would like to thank AMACOVID, Asociación Madrileña de Afectados por el COVID-19.
Author Contributions
Conceptualization: C.G.C., M.C.C., C.P.S. and R.S.P.; formal analysis: C.G.C., M.C.C., J.B.-L. and D.G.-A.; investigation: C.G.C., M.C.C., R.S.P., J.B.-L. and D.G.-A.; methodology, C.G.C. and M.C.C.; resources: C.G.C. and R.S.P.; software: M.C.C.; supervision: C.G.C.; validation: C.G.C., M.C.C., J.B.-L. and D.G.-A.; writing: C.G.C., M.C.C. and J.B.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by RoboCity2030-DIH-CM Madrid Robotics Digital Innovation Hub (“Robotica aplicada a la mejora de la calidad de vida de los ciudadanos, Fase IV”; S2018/NMT-4331), funded by Comunidad de Madrid and cofunded by Structural Funds of the EU. and partially funded by Universidad Politécnica de Madrid, Vicerrectorado de Estrategia Académica e Internacionalización. Area Cooperación al Desarrollo. J. Benito-León is supported by the National Institutes of Health, Bethesda, MD, USA (NINDS #R01 NS39422 and R01NS094607), European Commission (grant ICT-2011-287739, NeuroTREMOR), the Ministry of Economy and Competitiveness (grant RTC-2015-3967-1, NetMD—platform for the tracking of movement disorder), and the Spanish Health Research Agency (grant FIS PI12/01602 and grant FIS PI16/00451.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the University Hospital “12 de Octubre”. Protocol code 17/035. Date: 15 December 2020.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data sets generated during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
There is no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vidal M., Turner J., Bulling A., Gellersen H. Wearable eye tracking for mental health monitoring. Comput. Commun. 2012;35:1306–1311. doi: 10.1016/j.comcom.2011.11.002. [DOI] [Google Scholar]
- 2.Larrazabal A.J., Garcia Cena C.G., Martínez C.E. Video-oculography eye tracking towards clinical applications: A review. Comput. Biol. Med. 2019;108:57–66. doi: 10.1016/j.compbiomed.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 3.Molitor R.J., Ko P.C., Ally B.A. Eye movements in Alzheimer’s disease. J. Alzheimer’s Dis. 2015;44:1–12. doi: 10.3233/JAD-141173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noiret N., Carvalho N., Laurent É., Chopard G., Binetruy M., Nicolier M., Monnin J., Magnin E., Vandel P. Saccadic eye movements and attentional control in Alzheimer’s disease. Arch. Clin. Neuropsychol. 2018;33:1–13. doi: 10.1093/arclin/acx044. [DOI] [PubMed] [Google Scholar]
- 5.Yang Q., Wang T., Su N., Xiao S., Kapoula Z. Specific saccade deficits in patients with Alzheimer’s disease at mild to moderate stage and in patients with amnestic mild cognitive impairment. Age. 2013;35:1287–1298. doi: 10.1007/s11357-012-9420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufman L.D., Pratt J., Levine B., Black S.E. Antisaccades: A probe into the dorsolateral prefrontal cortex in Alzheimer’s disease. A critical review. J. Alzheimer’s Dis. 2010;19:781–793. doi: 10.3233/JAD-2010-1275. [DOI] [PubMed] [Google Scholar]
- 7.Crawford T.J., Higham S., Renvoize T., Patel J., Dale M., Suriya A., Tetley S. Inhibitory control of saccadic eye movements and cognitive impairment in Alzheimer’s disease. Biol. Psychiatry. 2005;57:1052–1060. doi: 10.1016/j.biopsych.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Pierrot-Deseilligny C., Milea D., Müri R.M. Eye movement control by the cerebral cortex. Curr. Opin. Neurol. 2004;17:17–25. doi: 10.1097/00019052-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Pretegiani E., Optican L.M. Eye movements in Parkinson’s disease and inherited parkinsonian syndromes. Front. Neurol. 2017;8:592. doi: 10.3389/fneur.2017.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fielding J., Kilpatrick T., Millist L., White O. Antisaccade performance in patients with multiple sclerosis. Cortex. 2009;45:900–903. doi: 10.1016/j.cortex.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Fielding J., Kilpatrick T., Millist L., White O. Multiple sclerosis: Cognition and saccadic eye movements. J. Neurol. Sci. 2009;277:32–36. doi: 10.1016/j.jns.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Clough M., Millist L., Lizak N., Beh S., Frohman T.C., Frohman E.M., White O.B., Fielding J. Ocular motor measures of cognitive dysfunction in multiple sclerosis I: Inhibitory control. J. Neurol. 2015;262:1130–1137. doi: 10.1007/s00415-015-7645-3. [DOI] [PubMed] [Google Scholar]
- 13.Piccardi L., Noris B., Barbey O., Billard A., Schiavone G., Keller F., von Hofsten C. Wearcam: A head mounted wireless camera for monitoring gaze attention and for the diagnosis of developmental disorders in young children; Proceedings of the RO-MAN 2007-the 16th IEEE International Symposium on Robot and Human Interactive Communication; Jeju, Korea. 26–29 August 2007; pp. 594–598. [Google Scholar]
- 14.Boraston Z., Blakemore S.J. The application of eye-tracking technology in the study of autism. J. Physiol. 2007;581:893–898. doi: 10.1113/jphysiol.2007.133587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorges M., Pinkhardt E.H., Kassubek J. Alterations of eye movement control in neurodegenerative movement disorders. J. Ophthalmol. 2014;2014:658243. doi: 10.1155/2014/658243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lage C., López-García S., Bejanin A., Kazimierczak M., Aracil-Bolaños I., Calvo-Córdoba A., Pozueta A., García-Martínez M., Fernández-Rodríguez A., Bravo-González M., et al. Distinctive Oculomotor Behaviors in Alzheimer’s Disease and Frontotemporal Dementia. Front. Aging Neurosci. 2021;12:525. doi: 10.3389/fnagi.2020.603790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shakespeare T.J., Kaski D., Yong K.X., Paterson R.W., Slattery C.F., Ryan N.S., Schott J.M., Crutch S.J. Abnormalities of fixation, saccade and pursuit in posterior cortical atrophy. Brain. 2015;138:1976–1991. doi: 10.1093/brain/awv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad I., Rathore F.A. Neurological manifestations and complications of COVID-19: A literature review. J. Clin. Neurosci. 2020;77:8–12. doi: 10.1016/j.jocn.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alonso-Lana S., Marquié M., Ruiz A., Boada M. Cognitive and neuropsychiatric manifestations of COVID-19 and effects on elderly individuals with dementia. Front. Aging Neurosci. 2020;12:369. doi: 10.3389/fnagi.2020.588872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roy D., Ghosh R., Dubey S., Dubey M.J., Benito-Leon J., Ray B.K. Neurological and neuropsychiatric impacts of COVID-19 pandemic. Can. J. Neurol. Sci. 2021;48:9–24. doi: 10.1017/cjn.2020.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghosh R., Biswas U., Roy D., Pandit A., Lahiri D., Ray B.K., Benito-León J. De novo movement disorders and COVID-19: Exploring the Interface. Mov. Disord. Clin. Pract. 2021;8:669–680. doi: 10.1002/mdc3.13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahalakshmi A.M., Ray B., Tuladhar S., Bhat A., Paneyala S., Patteswari D., Sakharkar M.K., Hamdan H., Ojcius D.M., Bolla S.R., et al. Does COVID-19 contribute to development of neurological disease? Immun. Inflamm. Dis. 2021;9:48–58. doi: 10.1002/iid3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onaolapo A., Onaolapo O. COVID-19, the Brain, and the Future: Is Infection by the Novel Coronavirus a Harbinger of Neurodegeneration? CNS & Neurol. Disord. Drug Targets. [(accessed on 5 January 2022)]. Available online: https://www.eurekaselect.com/article/119626. [DOI] [PubMed]
- 27.Pinna P., Grewal P., Hall J.P., Tavarez T., Dafer R.M., Garg R., Osteraas N.D., Pellack D.R., Asthana A., Fegan K., et al. Neurological manifestations and COVID-19: Experiences from a tertiary care center at the frontline. J. Neurol. Sci. 2020;415:116969. doi: 10.1016/j.jns.2020.116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varatharaj A., Thomas N., Ellul M.A., Davies N.W., Pollak T.A., Tenorio E.L., Sultan M., Easton A., Breen G., Zandi M., et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., et al. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaumont H., San-Galli A., Martino F., Couratier C., Joguet G., Carles M., Roze E., Lannuzel A. Mixed central and peripheral nervous system disorders in severe SARS-CoV-2 infection. J. Neurol. 2020;267:3121–3127. doi: 10.1007/s00415-020-09986-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2021;21:703–709. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Findlay J.M., Walker R. A model of saccade generation based on parallel processing and competitive inhibition. Behav. Brain Sci. 1999;22:661–674. doi: 10.1017/S0140525X99002150. [DOI] [PubMed] [Google Scholar]
- 33.Leigh J., Zee D.S. The Neurology of Eye Movements. Oxford University Press; Oxford, UK: 2015. [Google Scholar]
- 34.Pupil Labs Getting Started—Hardware. [(accessed on 28 February 2021)]. Available online: https://docs.pupil-labs.com/core/hardware/
- 35.Pupil Labs Pupil Core—Tech Specs. [(accessed on 1 March 2021)]. Available online: https://pupil-labs.com/products/core/tech-specs/
- 36.PsychoPy PsychoPy—Now Running Studies Online. [(accessed on 7 March 2021)]. Available online: https://www.psychopy.org/
- 37.Peirce J., Gray J.R., Simpson S., MacAskill M., Höchenberger R., Sogo H., Kastman E., Lindeløv J.K. PsychoPy2: Experiments in behavior made easy. Behav. Res. Methods. 2019;51:195–203. doi: 10.3758/s13428-018-01193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosimann U., Müri R., Burn D., Felblinger J., O’Brien J., McKeith I. Saccadic eye movement changes in Parkinson’s disease dementia and dementia with Lewy bodies. Brain. 2005;128:1267–1276. doi: 10.1093/brain/awh484. [DOI] [PubMed] [Google Scholar]
- 39.Jehangir N., Yu C.Y., Song J., Shariati M.A., Binder S., Beyer J., Santini V., Poston K., Liao Y.J. Slower saccadic reading in Parkinson’s disease. PLoS ONE. 2018;13:e0191005. doi: 10.1371/journal.pone.0191005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Antoniades C., Ettinger U., Gaymard B., Gilchrist I., Kristjánsson A., Kennard C., John-Leigh R., Noorani I., Pouget P., Smyrnis N., et al. An internationally standardised antisaccade protocol. Vis. Res. 2013;84:1–5. doi: 10.1016/j.visres.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 41.MacAskill M., Anderson T. Eye movements in neurodegenerative diseases. J. Neuro-Ophthalmol. Neuro-Otol. 2016;29:61–68. doi: 10.1097/WCO.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 42.Crawford T.J., Smith E.S., Berry D.M. Eye gaze and aging: Selective and combined effects of working memory and inhibitory control. Front. Hum. Neurosci. 2017;11:563–573. doi: 10.3389/fnhum.2017.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larrazabal A.J., Garcia Cena C.E., Martínez C.E. Eye corners tracking for head movement estimation; Proceedings of the IEEE International Work Conference on Bioinspired Intelligence (IWOBI 2019); Budapest, Hungary. 3–5 July 2019; pp. 1–5. [Google Scholar]
- 44.Garcia Cena C.E., Gomez Andres D., Pulido Valdeolivas I. Measurement and Analysis of Eye Movements Performance to Predict Healthy Brain Aging. IEEE Access. 2020;8:87201–87213. doi: 10.1109/ACCESS.2020.2992254. [DOI] [Google Scholar]
- 45.Garcia Cena C.E., Gómez Andrés D., Pulido Valdeoliva I., Acebrón López R., Espinoza Gómez R., Ramos Vázquez S. Device for Synchronized Measure of Ocular and Cephalic Movements. Nº EP3241488A1. European Patent. 2017 November 8;
- 46.Hernández E., Hernández S., Molina D., Acebrón R., García Cena C.E. OSCANN: Technical characterization of a novel gaze tracking analyzer. Sensors. 2018;18:522. doi: 10.3390/s18020522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kulke L., Atkinson J., Braddick O. Automatic Detection of Attention Shifts in Infancy: Eye Tracking in the Fixation Shift Paradigm. PLoS ONE. 2015;10:e0142505. doi: 10.1371/journal.pone.0142505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kano F., Hirata S., Call J., Tomonaga M. The visual strategy specific to humans among hominids: A study using the gap–overlap paradigm. Vis. Res. 2011;51:2348–2355. doi: 10.1016/j.visres.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 49.FKalesnykas R.P., Hallett P.E. The differentiation of visually guided and anticipatory saccades in gap and overlap paradigms. Exp. Brain Res. 1987;68:115–121. doi: 10.1007/BF00255238. [DOI] [PubMed] [Google Scholar]
- 50.Özyurt J., Greenlee M.W. Neural correlates of inter- and intra-individual saccadic reaction time differences in the gap/overlap paradigm. J. Neurophysiol. 2011;105:2438–2447. doi: 10.1152/jn.00660.2009. [DOI] [PubMed] [Google Scholar]
- 51.Bucci M.P., Pouvreau N., Yang Q., Kapoula Z. Influence of gap and overlap paradigms on saccade latencies and vergence eye movements in seven-year-old children. Exp. Brain Res. 2005;164:48–57. doi: 10.1007/s00221-005-2214-0. [DOI] [PubMed] [Google Scholar]
- 52.Takagi M., Frohman E.M., Zee D.S. Gap-overlap effects on latencies of saccades, vergence and combined vergence-saccades in humans. Vis. Re Res. 1995;35:3373–3388. doi: 10.1016/0042-6989(95)00073-N. [DOI] [PubMed] [Google Scholar]
- 53.Chehrehnegar N., Nejati V., Shati M., Esmaeili M., Rezvani Z., Haghi M., Foroughan M. Behavioral and cognitive markers of mild cognitive impairment: Diagnostic value of saccadic eye movements and Simon task. Aging Clin. Exp. Res. 2019;31:1591–1600. doi: 10.1007/s40520-019-01121-w. [DOI] [PubMed] [Google Scholar]
- 54.Polden M., Wilcockson T.D., Crawford T.J. The disengagement of visual attention: An eye-tracking study of cognitive impairment, ethnicity and age. Brain Sci. 2020;10:461. doi: 10.3390/brainsci10070461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Freitas Pereira M.L.G., de Arruda Camargo M.v.Z., Bellan A.F.R., Tahira A.C., Dos Santos B., Dos Santos J., Machado-Lima A., Nunes F.L., Forlenza O.V. Visual Search Efficiency in Mild Cognitive Impairment and Alzheimer’s Disease: An Eye Movement Study. J. Alzheimer’s Dis. 2020;75:261–275. doi: 10.3233/JAD-190690. [DOI] [PubMed] [Google Scholar]
- 56.Jin Z., Reeves A. Attentional release in the saccadic gap effect. Vis. Res. 2009;49:2045–2055. doi: 10.1016/j.visres.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 57.Pratt J., Lajonchere C.M., Abrams R.A. Attentional modulation of the gap effect. Vis. Res. 2006;46:2602–2607. doi: 10.1016/j.visres.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 58.Matsuo Y., Watanabe M., Taniike M., Mohri I., Kobashi S., Tachibana M., Kobayashi Y., Kitamura Y. Gap effect abnormalities during a visually guided pro-saccade task in children with attention deficit hyperactivity disorder. PLoS ONE. 2015;10:e0125573. doi: 10.1371/journal.pone.0125573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crawford T.J., Higham S., Mayes J., Dale M., Shaunak S., Lekwuwa G. The role of working memory and attentional disengagement on inhibitory control: Effects of aging and Alzheimer’s disease. Age. 2013;35:1637–1650. doi: 10.1007/s11357-012-9466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomas E.H., Myles J.B., Tan E.J., Neill E., Carruthers S.P., Sumner P.J., Bozaoglu K., Gurvich C. Working memory and attention influence antisaccade error rate in schizophrenia. J. Int. Neuropsychol. Soc. JINS. 2019;25:174–183. doi: 10.1017/S1355617718001066. [DOI] [PubMed] [Google Scholar]
- 61.Schut M.J., Van der Stoep N., Postma A., Van der Stigchel S. The cost of making an eye movement: A direct link between visual working memory and saccade execution. J. Vis. 2017;17:15–25. doi: 10.1167/17.6.15. [DOI] [PubMed] [Google Scholar]
- 62.Epelboim J., Suppes P. A model of eye movements and visual working memory during problem solving in geometry. Vis. Res. 2001;41:1561–1574. doi: 10.1016/S0042-6989(00)00256-X. [DOI] [PubMed] [Google Scholar]
- 63.Wenban-Smith M.G., Findlay J.M. Express saccades: Is there a separate population in humans? Exp. Brain Res. 1991;87:218–222. doi: 10.1007/BF00228523. [DOI] [PubMed] [Google Scholar]
- 64.Koller K., Rafal R.D. Saccade latency bias toward temporal hemifield: Evidence for role of retinotectal tract in mediating reflexive saccades. Neuropsychologia. 2019;128:276–281. doi: 10.1016/j.neuropsychologia.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 65.White B.J., Munoz D.P. The Superior Colliculus. Oxford University Press; New York, NY, USA: 2011. Oxf. Handb. Eye Movements. [Google Scholar]
- 66.Reuter B., Kathmann N. Using saccade tasks as a tool to analyze executive dysfunctions in schizophrenia. Acta Psychol. 2004;115:255–269. doi: 10.1016/j.actpsy.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 67.Hutton S., Ettinger U. The antisaccade task as a research tool in psychopathology: A critical review. Psychophysiology. 2002;43:302–313. doi: 10.1111/j.1469-8986.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- 68.Ettinger U., Kumari V., Crawford T.J., Corr P.J., Das M., Zachariah E., Hughes C., Sumich A.L., Rabe-Hesketh S., Sharma T. Smooth pursuit and antisaccade eye movements in siblings discordant for schizophrenia. J. Psychiatr. Res. 2004;38:177–184. doi: 10.1016/S0022-3956(03)00105-5. [DOI] [PubMed] [Google Scholar]
- 69.Smyrnis N., Evdokimidis I., Stefanis N., Constantinidis T., Avramopoulos D., Theleritis C., Paximadis C., Efstratiadis C., Kastrinakis G., Stefanis C. The antisaccade task in a sample of 2.006 young males. Effects of task parameters. Exp. Brain Res. 2002;147:53–63. doi: 10.1007/s00221-002-1207-5. [DOI] [PubMed] [Google Scholar]
- 70.Jóhannesson Ó.I., Tagu J., Kristjánsson Á. Asymmetries of the visual system and their influence on visual performance and oculomotor dynamics. Eur. J. Neurosci. 2018;48:3426–3445. doi: 10.1111/ejn.14225. [DOI] [PubMed] [Google Scholar]
- 71.Bargary G., Bosten J.M., Goodbourn P.T., Lawrance-Owen A.J., Hogg R.E., Mollon J. Individual differences in human eye movements: An oculomotor signature? Vis. Res. 2017;141:157–169. doi: 10.1016/j.visres.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 72.Ettinger U., Antonova E., Crawford T.J., Mitterschiffthaler M.T., Goswani S., Sharma T., Kumari V. Structural neural correlates of prosaccade and antisaccade eye movements in healthy humans. NeuroImage. 2005;24:487–494. doi: 10.1016/j.neuroimage.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 73.Jernajczyk W., Sobańska A., Czerwosz L., Szatkowska E. The influence of age and gender on the latency of eye movement in healthy humans. J. Physiol. Pharmacol. 2005;56:93–100. [PubMed] [Google Scholar]
- 74.Lai H.Y., Saavedra-Peña G., Sodini C.G., Sze V., Heldt T. Measuring Saccade Latency Using Smartphone Cameras. IEEE J. Biomed. Health Inform. 2020;24:885–897. doi: 10.1109/JBHI.2019.2913846. [DOI] [PubMed] [Google Scholar]
- 75.Israël I. Memory-guided saccades: What is memorized? Exp. Brain Res. 1992;90:221–224. doi: 10.1007/BF00229275. [DOI] [PubMed] [Google Scholar]
- 76.Brown M., DeSouza J., Goltz H.C., Ford K., Menon R.S., Goodale M.A., Everling S. Comparison of Memory- and Visually Guided Saccades Using Event-Related fMRI. J. Neurophysiol. 2004;91:873–889. doi: 10.1152/jn.00382.2003. [DOI] [PubMed] [Google Scholar]
- 77.Bonnet C., Hanuška J., Rusz J., Rivaud-Péchoux S., Sieger T., Majerová V., Serranová T., Gaymard B., Růžička E. Horizontal and vertical eye movement metrics: What is important? Clin. Neurophysiol. 2013;124:2216–2229. doi: 10.1016/j.clinph.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 78.Azri M., Young S., Lin H., Tan C., Yang Z. Diagnosis of Ocular Myasthenia Gravis by means of tracking eye parameters; Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; Chicago, IL, USA. 26–30 August 2014; pp. 1460–1464. [DOI] [PubMed] [Google Scholar]
- 79.Tagu J., Doré-Mazars K., Vergne J., Lemoine-Lardennois C., Vergilino-Perez D. Recentering bias for temporal saccades only: Evidence from binocular recordings of eye movements. J. Vis. 2018;18:10. doi: 10.1167/18.1.10. [DOI] [PubMed] [Google Scholar]
- 80.Pierrot-Deseilligny C.H., Müri R.M., Nyffeler T., Milea D. The role of the human dorsolateral prefrontal cortex in ocular motor behavior. Ann. N. Y. Acad. Sci. 2005;1039:239–521. doi: 10.1196/annals.1325.023. [DOI] [PubMed] [Google Scholar]
- 81.Pierrot-Deseilligny C., Müri R.M., Rivaud-Pechoux S., Gaymard B., Ploner C.J. Cortical control of spatial memory in humans: The visuooculomotor model. Ann. Neurol. 2002;52:10–19. doi: 10.1002/ana.10273. [DOI] [PubMed] [Google Scholar]
- 82.Ouerfelli-Ethier J., Salemme R., Fournet R., Urquizar C., Pisella L., Khan A.Z. Impaired Spatial Inhibition Processes for Interhemispheric Anti-saccades following Dorsal Posterior Parietal Lesions. Cereb Cortex Commun. 2021;2:tgab054. doi: 10.1093/texcom/tgab054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johnston K., Everling S. Neurophysiology and neuroanatomy of reflexive and voluntary saccades in non-human primates. NeuroImage. 2008;68:271–283. doi: 10.1016/j.bandc.2008.08.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during the current study are available from the corresponding author upon reasonable request.