Abstract
Bionanotechnology is a branch of science that has revolutionized modern science and technology. Nanomaterials, especially noble metals, have attracted researchers due to their size and application in different branches of sciences that benefit humanity. Metal nanoparticles can be synthesized using green methods, which are good for the environment, economically viable, and facilitate synthesis. Due to their size and form, gold nanoparticles have become significant. Plant materials are of particular interest in the synthesis and manufacture of theranostic gold nanoparticles (NPs), which have been generated using various materials. On the other hand, chemically produced nanoparticles have several drawbacks in terms of cost, toxicity, and effectiveness. A plant-mediated integration of metallic nanoparticles has been developed in the field of nanotechnology to overcome the drawbacks of traditional synthesis, such as physical and synthetic strategies. Nanomaterials′ tunable features make them sophisticated tools in the biomedical platform, especially for developing new diagnostics and therapeutics for malignancy, neurodegenerative, and other chronic disorders. Therefore, this review outlines the theranostic approach, the different plant materials utilized in theranostic applications, and future directions based on current breakthroughs in these fields.
Keywords: gold, cytotoxicity, antioxidant, anticancer, antibacterial, antifungal
1. Introduction
Gold nanoparticles (AuNPs), due to their unique qualities and various surface characteristics, have been widely exploited in bionanotechnology. The ease with which AuNPs can be functionalized makes them a flexible platform for nano biological assemblies containing oligonucleotides [1], antibodies [2], and proteins [3]. AuNPs bioconjugates have also emerged as attractive options for developing novel biomaterials for biomedical research. The versatility of AuNPs has made them useful in various biomedical applications. The binding of the sample to the AuNPs can change the rheological feature of AuNPs, such as surface plasmon resonance, conductivity, and redox behavior, resulting in notable signals [4,5,6,7] in diagnostics. With their enormous surface area, AuNPs can also be used as a platform for therapeutic agents. Nanotechnology has been in existence for thousands of years. Ancient people used to stain their drinking glasses with nanoparticles [8]. The divergence of nanotechnology within other fields of science and further innovations have made a significant impact on biotechnology, medicine, pharmaceutics, physics, chemistry, and optics, etc. There is evidence that metals are present in living systems in different forms, playing a significant role in various biochemical processes, growth metabolism, and healing [9]. Blood contains the Hema protein, Zn, Mn, Cu, and other vital trace metals in the biological system.
This review aims to summarize the data on gold nanoparticles synthesized by extracts of medicinal plants, their parts, and their usefulness in biological and theranostic properties.
1.1. Nanoparticle Synthesis
Nanoparticle synthesis from metals has gained enormous interest among researchers because of nanoparticles’ diverse application in many fields such as cancer therapy, drug delivery, food safety, fabrics, chemistry, water treatment, and photocatalysis, as well as because of their antioxidant, antibacterial, and cytotoxic properties [10,11,12,13,14,15,16,17,18,19,20]. The uses of nanoparticles in various fields are possible because of several factors, including the nanoparticles′ shape, size, distribution, and surface plasmon [21,22,23].
Nanoparticles have been used for thousands of years without knowledge concerning the exact phenomenon and synthesis [8]. Drinking glasses in ancient times were coated with Au nanoparticles and were synthesized following three primary methods: physical, chemical, and biological methods.
1.2. Physical Method
The advantages of the physical synthesis method are the absence of a solvent, which is hazardous to the environment, and the uniformity of the nanoparticles produced by the physical methods. The tube furnace method of synthesis occupies an ample space, and an enormous quantity of heat is required to raise the temperature of the furnace. Several minutes are necessary to preheat the furnace [24]. A small ceramic heater with a local heating chamber could be used to synthesize Ag nanoparticles [25]. As a result, the formed nanoparticles were reported to have a mean geometric diameter that was spherical without agglomeration [26]. The advantage of the laser ablation method, in comparison to other techniques, is that it is free from chemical reagents. The purity of the nanoparticles was assured in this method [27]. The nanoparticles produced by the discharge method used to fabricate Ag nanoparticles [28,29] had 99.99% purity. The purity and size distribution were uniform when compared to other forms of synthesis.
1.3. Chemical Synthesis of Gold Nanoparticles
In recent years, a solution-based strategy for controlling the size, shape, and surface functionality has been created [30,31,32]. In 1951, a new method for synthesizing AuNPs was devised by boiling hydrogen tetrachloroaurate (HAuCl4) with citric acid [33]. Citrate has a lowering and stabilizing effect [34]. To adjust particle size, Frens developed the process by modifying the gold-to-citrate ratio [33]. This approach has been commonly used to make dilute solutions of relatively stable spherical AuNPs with diameters of 10 to 20 nm; however, bigger AuNPs (e.g., 100 nm) have also been made. These citrate-stabilized AuNPs may undergo irreversible aggregation during the functionalization process with thiolate ligands. Several solutions have been devised to tackle this difficulty, including using a biosurfactant, Tween 20. Similarly, a two-step method for functionalizing gold nanoparticles was made by reducing tetrachloroauric acid in water with trisodium citrate. The physisorbed chloride and citrate on gold nanoparticles are first displaced by thioctic acid, which is then replaced by thiols with the desired functionality in the second step [35,36]. The demand for high dilution, on the other hand, makes large-scale manufacture difficult.
AuNPs synthesis [37] was conducted in 1994 to produce organic soluble alkanethiol-stabilized AuNPs by adopting a biphasic reduction, with the use of tetraoctylammonium bromide as a phase transfer reagent and sodium borohydride (NaBH4) as a reducing agent [37]. By changing response variables such as the gold-to-thiol ratio, the reduction rate, and the reaction temperature, this technique yields low-dispersity AuNPs ranging from 1.5 to 5 nm [38]. The synergic impact of thiol-gold generated strong connections and Van der Waals attractions between the adjacent ligands, giving these alkanethiol-protected AuNPs better stability than most other AuNPs [39].
2. Biological Method of Synthesis
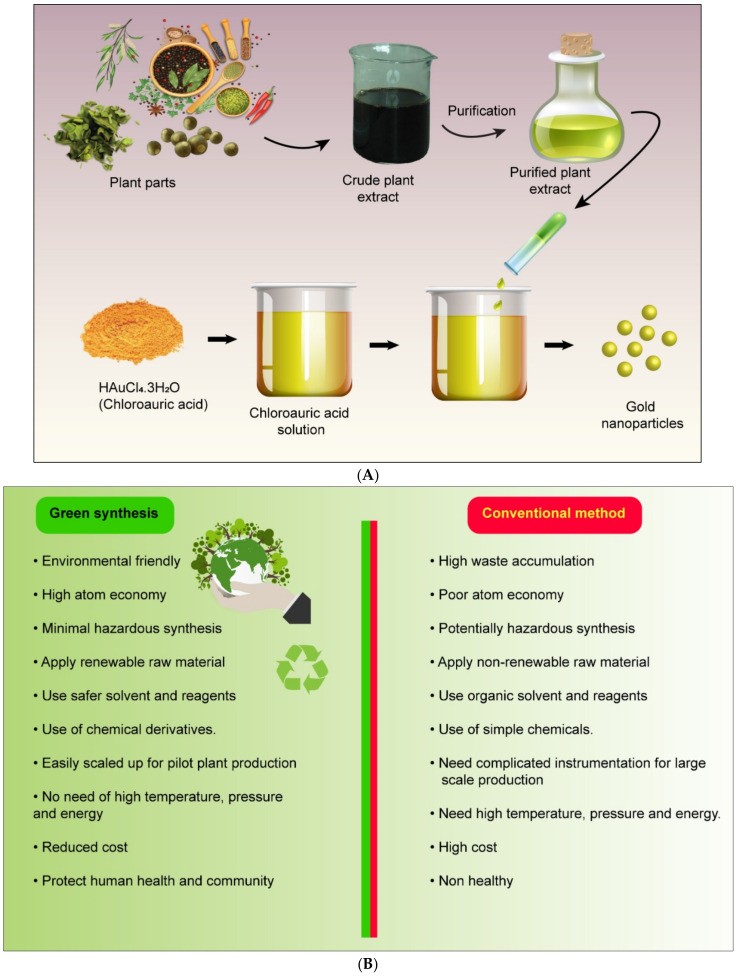
Although the chemical synthesis of metallic nanoparticles is a standard procedure, the cost and hazardous effects of reducing reagents and stabilizing agents restrict their use. Furthermore, in biomedical applications, these nanoparticles could be toxic [40,41]. As a result, ecologically friendly and cost-effective nanoparticle synthesis techniques that do not rely on harmful chemicals are needed. In recent years, biological nanoparticle production has gained popularity as a green and environmentally friendly process [42]. Plants or plant extracts and microorganisms and enzymes were employed to synthesize nanoparticles using a natural method [43,44]. The proposed synthetic mechanism for plant-mediated synthesis of gold nanoparticles is depicted in Figure 1A.
Figure 1.
(A) Proposed synthetic mechanism for plant-mediated synthesis of gold nanoparticles. (B) The advantages of green synthesis over conventional methods.
Plants are increasingly being used to synthesize nanoparticles because of their widespread availability, low cost, environmental friendliness, and non-toxic nature. Plants such as Azadirachta indica have recently been used to study the production of AuNPs. Medicago sativa, Aloe vera, Cinnamomum camphora, Pelargonium graveolens, Coriandrum sativum, Coriandrum sativum, Lemongrass, Terminalia catappa, and Terminalia catappa have all been reported [41,45,46,47,48,49,50,51].
Many scientists are experimenting with the production of AuNPs from plant extracts as biomedicines against drug-resistant bacteria. Arunachalam et al., 2013, proposed using Memecylon umbellatum nanoparticles as chemical sensors [52]. Kalishwaralal et al., 2010, showed how a bacterium, Brevibacterium casei, can synthesize and stabilize spherical-shaped Au and Ag nanoparticles in an unprecedented green process. The biological activities of the produced particles were confirmed based on their durable anti-coagulant actions. Similarly, Citrus limon, Citrus reticulata, and Citrus sinensis, all citrus fruits, as well as Piper pedicellatum, have been synthesized as polymorphic gold nanoparticles with promising biological uses. These chemical constituents can operate as a reducing, stabilizing, and capping agent [52,53,54,55,56,57]. Chebula Terminalia, Memecylon edule, and Nyctanthes arbor-tristis flower extract have potential medicinal and industrial applications. Murraya koenigii and Musa paradisiaca show antibacterial activity; Mangifera indica, Cochlospermum gossypium, and Cinnamomum zeylanicum photoluminescent particles are used for the production of noble metal nanoparticles, which enable much faster synthesis and colloidal stability comparable to those of chemical reduction [58,59,60,61,62,63,64,65,66,67].
2.1. Green Synthesis of Gold Nanoparticles
Many metal nanoparticles synthesized via the green process possess several advantages, as shown in Figure 1B. Their unique physicochemical properties, high surface-to-volume ratio, low cost of synthesis, and surface functionalization were reported by Ankit Kumar Singh. Additionally, this review found that several studies have reported in detail a variety of plants and plant parts used in metal nanoparticle generation: the bark of Mimusops elengi was used to synthesize Au nanoparticles; bimetallic nanoparticles were synthesized using Azadictira Indica leaf extract; Au nanoparticles were synthesized from natural rubber; and Aelovera plant extract and lemongrass extract have applications on infrared-absorbing coating [68,69,70,71,72,73]. The antioxidant, anti-inflammatory, antidiabetic, and antibacterial activities of Holopetelea integrifolia leaf extract were studied, and synthesized Au nanoparticles from Halymenia dilatata were studied regarding their antioxidant, anticancer, and antibacterial activities; synthesized conjugated Au nanoparticles from Nerium oleander were studied regarding their anticancer activity against MCR-7 cell lines [74,75,76]. The anticancer activity of Au nanoparticles synthesized using Lonicera japonica was also studied. Ag and Au nanoparticles synthesized from Pleuropterus multiflorus roots were investigated regarding their anticancer activity against the A549 lung cancer cell line [77,78]. Au nanoparticles synthesized using the Mucuna pruriens plant extract were studied regarding an antiparkinsonian drug, and it was reported that poly-shaped Au nanoparticles were synthesized using Saraca indica bark extract and were studied regarding catalytic reduction. The anticancer activity of Ag and Au nanoparticles synthesized using Dendropanax morbifera leaf extract was studied, as well as the antimicrobial characteristics of Au and Ag nanoparticles using Trianthema decandra extract. The antioxidant and anticancer properties of Au nanoparticles synthesized using Antigonon leptopus leaf extract were studied, and the anticancer activities of noble metal nanoparticles using Psidium guajava leaf extract and Syzygium aromaticum bud extract were studied. The antibacterial properties of Au nanoparticles synthesized from Nepenthes khasiana leaf extract were investigated, as well as Au nanoparticles synthesized from Schisandra Chinensis fruit extract. Ag and Au nanoparticles synthesized using Dalbergia sissoo leaf extract were studied, and Ag nanoparticles synthesized from Cassia italica leaf extract were also studied. The kinetics of the Au nanoparticles synthesized using Camellia chinesis leaves and leaf buds were studied, as well as the apoptotic effects of Au nanoparticles synthesized using Curcuma wenyujin [79,80,81,82,83,84,85,86,87].
2.2. Medicinal Plants
Nature’s contribution to the health of human beings is unimaginable. A wide variety of plants are used in curing diseases and for a healthy lifestyle. India has a rich source of medicinal plants used for various purposes. More than 17,000 species are used as medicinal plants in India. The constituents/drugs present in medicinal plants are called phytochemicals. These phytochemicals act on the biochemical processes in animals, human beings, and microbes. The properties of phytochemicals are used due to their antioxidant, antimicrobial, and anti-inflammatory properties [88,89,90].
The World Health Organization (WHO) indicated that traditional remedies are used by 80% of the world’s population. For a long time, plants have been used as medicine in India’s alternative medical systems, such as Unani, Ayurveda, Siddha, Yoga, and homeopathy. Plant-derived medicines are alternatives to synthetic drugs, gaining importance in modern medicine. In the developing world, primary health care services have benefited from medicinal plants. In the Ayurvedic medical system, many plants and plant-based materials are employed to treat ailments. A treatise on Ayurvedic medicine called “Charaka Samhitha” mentions over 700 herbs [91,92,93,94,95,96,97]. Several therapeutic plants are mentioned in the Vedas, such as the Rig Veda and the Atharva Veda.
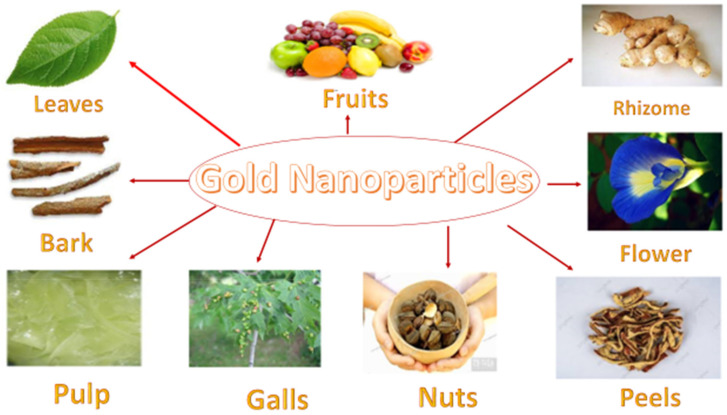
AuNPs are well-known nanomaterials with a wide range of biomedical applications. AuNPs can be synthesized using a variety of microbes and plants, mainly through the use of fruit extracts. Fruit extracts are used because they naturally concentrate chemicals with medicinal effects. Studies have shown that UV–visible spectroscopy, transmission or scanning electron microscopy, dynamic light scattering, and Fourier transformation infrared spectroscopy techniques are the methods most often used to characterize AuNPs and capping biomaterial. Figure 2 shows some of the important outcomes in gold nanoparticles obtained from plant components.
Figure 2.
Plant parts extract can be used for the biosynthesis of gold nanoparticles.
3. Characterization
3.1. UV–Visible Spectroscopic Analysis
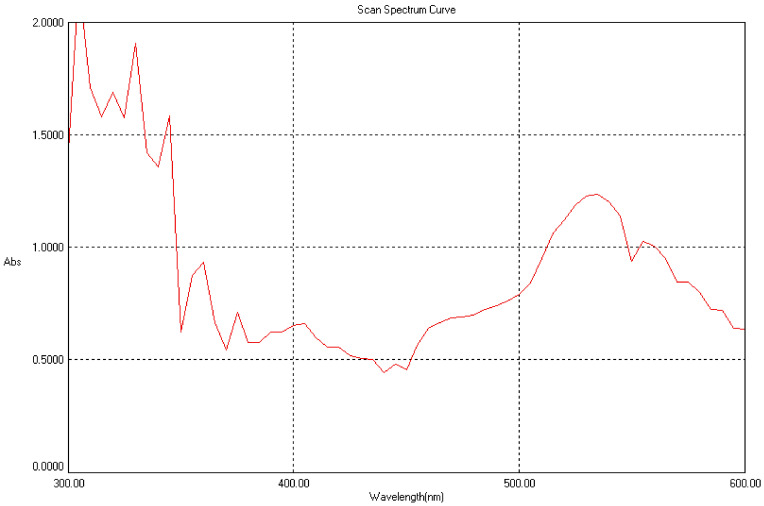
In an aqueous solution, gold nanoparticles synthesized from various plant parts were measured using a UV photometer and a Lab India UV3000 spectrophotometer, which read at 450 nm and 650 nm for the Au nanoparticles. Readings were taken every 30 min for 6 h. The absorbance and transmittance of the Au nanoparticles were measured at 450 nm to 650 nm using 3 mL of each sample in a cuvette, and they were subjected to spectral analysis. At 520–560 nm, a single, narrow absorbance band was found, which is typical of the production of tiny gold nanoparticles [98,99,100,101,102,103], and this was validated by the TEM results as shown in Figure 3.
Figure 3.
UV-spectral analysis for gold nanoparticles.
3.2. Fourier Transform Infrared Spectroscopic Analysis (FTIR)
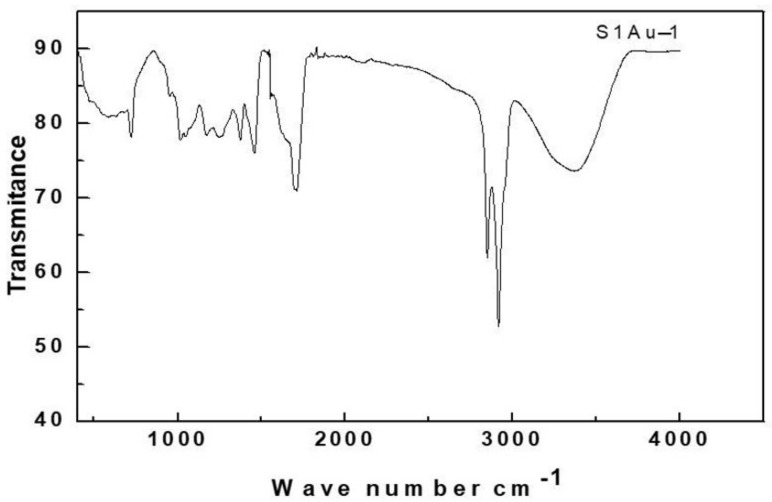
A total of 5 gm of each synthesized freeze-dried Au nanoparticle from different plants parts was taken and pressed with 0.2 gm of KBr pellets to measure the infrared radiation spectrum (IR) examined under an FTIR spectrophotometer (JASCO) over wavelengths in the range of 4000 cm−1–400 cm−1. The FTIR spectrum of the green synthesized AuNPs is shown in Figure 4. The strong bands at 3389 cm−1 (O-H stretching alcohol), 2919 cm−1 (C-H stretching alkane) and 2844 cm−1 (C-H stretching aldehyde) were due to the reduction of Au3+ to Au0. A band at 1458 cm−1 corresponds to an NH bend, and the very broad band of NH+3 stretch was observed in the 3000–3500 cm−1 range. The peaks at 1700 cm−1 (C-C stretching alkane), 1374 cm−1 (O-H bending phenol), and 1162 cm−1 (CO-O- CO stretching anhydride) confirm the capping biomaterials of phytochemicals from plant extracts such as polyphenols, flavonoids, and terpenoid compounds. Similar reports of FTIR peaks for phenols and flavonoids from gold nanoparticles biosynthesized from Cissus quadrangular extract confirm the capping biomaterial of the synthesized nanoparticles. The bands at 1261 cm−1 and 1034 cm−1 are typically assigned to the vibration of ribose (C-C sugar), which correspond to an epoxy bond, semi-acetal, and primary alcohol, respectively. Further, the bands at 2920 and 1374 cm−1 correspond to methylene stretching and methyl deformation vibrations, respectively.
Figure 4.
FTIR for gold nanoparticles.
For example, the gold nanoparticles produced using plant extracts had bands at 617 cm−1, 1125 cm−1, 1376 cm−1, 1658 cm−1, and 3278 cm−1 in their FTIR pattern [104,105,106,107,108,109]. The aromatic hydroxyl and benzene rings were assigned bands at 3402 cm−1, 1606 cm−1, and 1518 cm−1, indicating that the extract contains phenols. The bands at 2931 cm−1 and 1402 cm−1 correspond to methylene stretching and methyl deformation vibrations, respectively, whereas the sugar content is shown by bands at 1260 cm−1, 1113 cm−1, and 1076 cm−1, which correspond to an epoxy bond, semi-acetal, and primary alcohol, respectively.
3.3. Transmission Electron Microscope (TEM)
The synthesized Au nanoparticles were loaded separately into the FEI. A Tecnai G2 F20 STFE-TEM microscope was used. The sample was dried by pressing with blotting paper to remove excess water and loaded onto the carbon-coated copper grid. The TEM was operated at 200 Kv, with a resolution of 0.24 nm, and Cs of 1.2 nm; the shape and size were determined as shown in Figure 5. The high-resolution TEM images show agglomerated polycrystalline particles, and the SAED confirmed the face-centered cubic (FCC) structure incorporation of the poly-dispersed XRD pattern. The EDX analysis proved the presence of only Au metal, and no other elements were present.
Figure 5.
TEM analysis for gold nanoparticles.
SEM, TEM, and AFM are the most commonly utilized microscopic techniques for morphological analyses of nanoparticles. The application of these microscopic methods in nanoparticle morphology research has already been mentioned. TEM has a higher magnification and resolution than the SEM. The electron diffraction pattern for a specified region (SAED) is also utilized in TEM to distinguish crystalline structures from amorphous structures [105,110]. The shape of the gold nanoparticles is studied using AFM [109,110,111].
3.4. X-ray Diffraction (XRD)
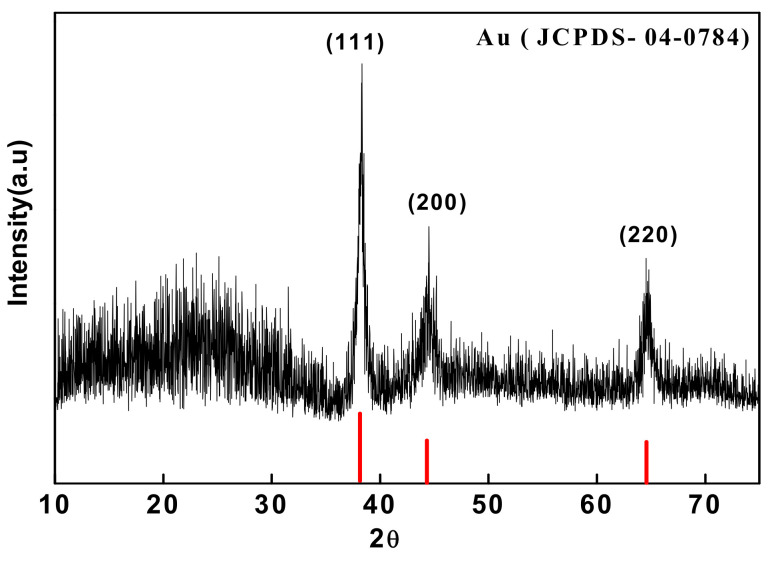
The Analytical Expert MRD, the model instrument, is generally utilized to investigate the characteristics of synthetic Au nanoparticles for samples. The fine powder of nanoparticles is loaded onto the XRD sample holder separately, and readings are recorded. The size of the Au nanoparticles is calculated using Debye-Scherer’s equation: D = 0.9λ/βcos θ, where D is the average crystallite size. X is the XRD wavelength (1.54 nm); Β is the (FWHM), and θ is the Bragg angle. The plant-mediated synthesized XRD characterized the Au nanoparticles. The diffraction peak 2θ values assigned at 38.2º, 44.4º, and 64.6º were denoted as the (111), (200), and (220) planes, respectively. The planes agree well with the JCPDS card: 04-0784 data. The XRD pattern determined the intensity of the peak, the peak position, the width, and the full width at half-maximum (FWHM) as shown in Figure 6. The XRD data revealed that the nanoparticles are crystalline and face-cantered cubic (fcc). The particle mean size was determined using Debye-Scherer’s formula to determine the average size of the Au particles. The high-energy X-rays can penetrate the materials deeply and reveal important details about the bulk structure. The Debye-Scherrer equation computes the crystallite sizes using the XRD technique. The usage of XRD patterns/peaks during gold nanoparticle production has been reported [98,104,110,111,112,113,114].
Figure 6.
XRD analysis for gold nanoparticles.
4. Theranostic Applications
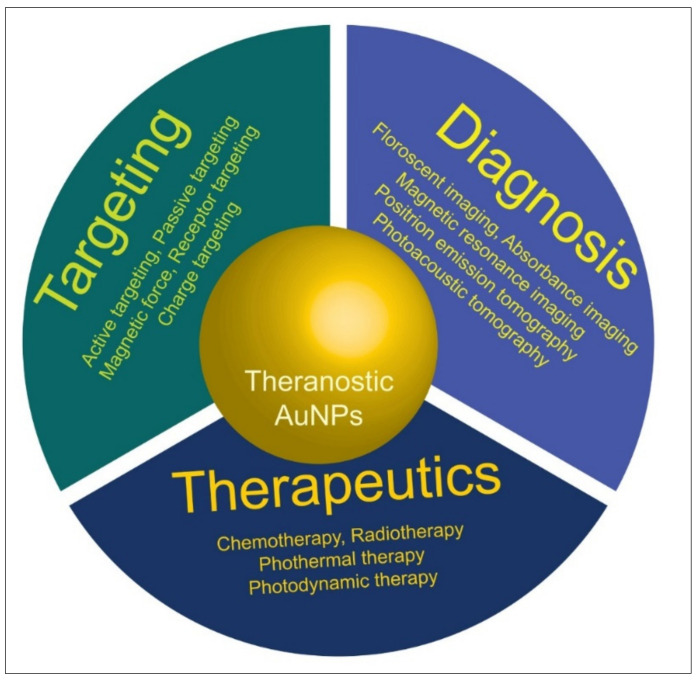
Nanoparticles are associated with a small stature and shape. The surface-to-volume ratio of nanoparticles is very high, which leads to enhanced electrical, optical, magnetic, antifungal, antioxidant, antibacterial, anti-inflammatory, and anticancer properties [115,116]. The surface-to-volume ratio offers many perspectives for the food sector. Nanoparticles’ are critical and significant in applications in biomedicine, especially in treating cancer, the diagnosis of HIV, and the proliferation of cancer cells. In 1918, scientists made drastic progress in finding the function of various metal nanoparticles in biological systems [117,118,119]. Metal nanoparticles, mainly gold, are used in medicine for diagnosis, targeting, and therapeutics (Figure 7).
Figure 7.
Theranostic applications of AuNPs in medical sciences and applied fields.
4.1. Principle of MTT Assay
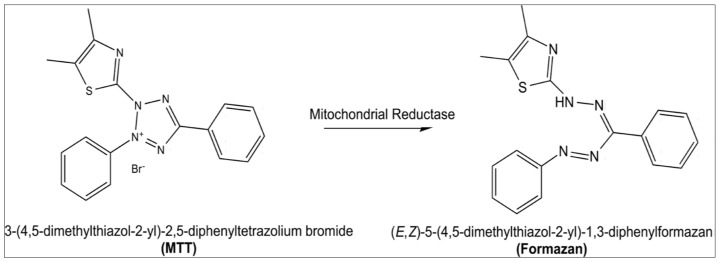
Tetrazolium salt reduction is now universally acknowledged as a reliable method of examining cell growth. MTT (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide) is a yellow tetrazolium reduced by metabolically active cells, in part via dehydrogenase enzymes, to generate reducing equivalents such as NADH and NADPH. The intracellular purple formazan that results can be solubilized and measured using spectrophotometric methods (Figure 8). The assay evaluates the cell proliferation rate and, conversely, cell viability reduction induced by metabolic processes such as apoptosis or necrosis [120].
Figure 8.
Principle of MTT assay.
4.2. Biological and Theranostic Applications
As shown in Table 1, many researchers have demonstrated that AuNPs can successfully attack cancer cells. AuNPs derived from Gymnema Sylvestre, often known as cowplant, were cytotoxic to Hep2 cells. After treatment with AuNPs, Hep2 cells showed morphological alterations. Increases in reactive oxygen species levels and alterations in the nucleus were discovered, implying that apoptosis was responsible for the demise of Hep2 cells [121]. Another cervical cancer cell type, the HeLa cell line, also reacted to AuNPs. Rounding, shrinkage, and granulation were identified as morphological alterations. The AuNPs’ activity was attributable to the NPs’ ability to penetrate the cell membrane efficiently. AuNPs have caused responses from other tumor cells, including Ehrlich’s ascites carcinoma, breast cancer cells, and MCF-7 cells. Green tea polyphenols were used in the production of AuNPs. AuNPs synthesized from green tea and AuNPs synthesized from epigallocatechin-3-gallate were compared. Both AuNPs were able to trigger apoptosis in tumor cells while preventing tumor cell damage in normal hepatocytes. Green-generated AuNPs, on the other hand, demonstrated improved tumoricidal and hepatoprotective effects. When AuNPs generated by Actinidia deliciosa were examined on HCT-116 cells using an MTT assay, they showed 71 percent activity at their highest concentration (350 g/mL). The cytotoxic effect of the AuNPs was shown to be concentration dependent [122].
Table 1.
Biological and theranostic applications of gold nanoparticles.
| S. No | Name of the Plant | Activity | Cell Line Used | Shape | Size (nm) | Ref. |
|---|---|---|---|---|---|---|
| 1 | Nanoparticles with antibacterial activity | |||||
| 1.1 | Areca catechu | Antibacterial | - | Spherical | 13 | [123] |
| 1.2 | Acorus calamus | Antibacterial | - | Spherical | 100 | [124] |
| 1.3 | Ananas comosus | Antibacterial | - | Spherical | 16 | [125] |
| 1.4 | Benincasa hispida | Antibacterial | - | Spherical | 23 | [126] |
| 1.5 | Brazilian red propolis | Antibacterial | - | Rods, triangular, pentagonal, hexagonal | 8–15 | [127] |
| 1.6 |
Clitoria ternatea (Asian
pigeonwings) |
Antibacterial | - | Spherical, triangular, hexagonal | 10 | [128] |
| 1.7 | Citrus maxima | Antibacterial | - | Spherical | 27–30 | [104] |
| 1.8 | Coreopsis lanceolate | Detections of aflatoxins | - | Sphere | 23–30 | [129] |
| 1.9 | Caesalpinia pulcherrima | Antibacterial | - | Spherical | 10–50 | [130] |
| 1.10 | Carthamus tinctorius L | Antibacterial | - | Triangular, spherical | 40–200 | [131] |
| 1.11 | Catharanthus roseus | Antibacterial | - | Spherical, triangular | 3–9 | [132] |
| 1.12 | Carica papaya | Antibacterial | - | Spherical, triangular | 2–20 | [133] |
| 1.13 | Coleus forskohlii | Bactericidal activity | - | Triangular | 25–40 | [134] |
| 1.14 | Ceiba pentandra (L) | Antibacterial | - | Spherical | 20–48 | [135] |
| 1.15 | Diospyros ferrea | Antibacterial | - | Diverse | 70–90 | [136] |
| 1.16 | Dioscorea batatas | Antibacterial | - | Diverse | 19–56 | [137] |
| 1.17 | Dimocarpus longan | Antibacterial | - | Diverse | 25 | [138] |
| 1.18 | Dracocephalum kotschyi | Antibacterial | - | Spherical | 11 | [139] |
| 1.19 | Euphorbia hirta | Antibacterial | - | Spherical | 6–7 | [140] |
| 1.20 | Gloriosa superba | Antibacterial | - | Spherical | 25 | [141] |
| 1.21 | Galaxaura elongate | Antibacterial | - | Rod, triangular, hexagonal | 3–77 | [142] |
| 1.22 | Bay cedar | Antibacterial | - | Spherical | 20–25 | [143] |
| 1.23 | Hibiscus cannabinus | Antibacterial | - | Spherical | 13 | [144] |
| 1.24 | Hoveniadulcis | Antibacterial | - | Spherical | 20 | [145] |
| 1.25 | Helianthus annuus | Antibacterial | - | Polydispersed | 35 | [146] |
| 1.26 | Hevea brasiliensis | Cytotoxicity and genotoxicity |
CHO-K1 cells | Spherical, triangular | 50 | [147] |
| 1.27 | Justica wynaadensis | Antibacterial | - | Spherical | 30–50 | [148] |
| 1.28 | Jasminum auriculatum | Antibacterial | - | Spherical | 8–37 | [149] |
| 1.29 | Lobila nicotianifolia | Antibacterial | - | Spherical | 80 | [150] |
| 1.30 | Mammea suriga | Antibacterial | - | Square | 50 | [151] |
| 1.31 | Mentha piperita | Antibacterial | - | Hexagonal | 78 | [152] |
| 1.32 | Maytenus royleanus | Antibacterial, Leshmenia | - | Hexagonal | 30 | [153] |
| 1.33 | Musa paradisiaca (Banana) | Antibacterial | - | Diverse | 300 | [154] |
| 1.34 | Nepenthes khasiana | Antibacterial | - | Spherical | 50–80 | [155] |
| 1.35 | Nigella arvensis | Antibacterial | - | Spherical | 3–37 | [156] |
| 1.36 | Punica granatum | Antibacterial | - | Spherical | 5.20 | [157] |
| 1.37 | Pistacia integerrima | Antibacterial | - | Granular | 20–200 | [158] |
| 1.38 | Plumeria alba | Antibacterial | - | Spherical | 16–28 | [159] |
| 1.39 | Platycodon grandiflorum | Antimicrobial | - | Spherical | 15 | [160] |
| 1.40 | Rivea hypocrateriformis | Antibacterial | - | Spherical | 10–50 | [161] |
| 1.41 | Solanum nigrum | Antibacterial | - | Spherical | 50 | [162] |
| 1.42 | Salicornia brachiate | Antibacterial | - | Polydispersed | 22–35 | [163] |
| 1.43 | Solanum lycopersicums | Antibacterial | - | Diverse | 14 | [164] |
| 1.44 | Trichoderma sp | Antibacterial | - | Pseudospheric | 1–24 | [165] |
| 1.45 | Trianthema decandra L | Antibacterial | - | Spherical, hexagonal, cuboidal | 38–80 | [166] |
| 1.46 | Zingiber officinale (Ginger) | Antibacterial | - | Spherical | 5–15 | [167] |
| 1.47 | Zizyphus mauritiana | Antibacterial | - | Spherical | 20–40 | [168] |
| 2 | Nanoparticles with Anticancer activity | |||||
| 2.1 | Areca catechu | Anticancer, catalyst | HeLa | Spherical | 13 | [123] |
| 2.2 | Artocarpus hirsutus (Wild jack) | Anticancer | HeLa, RKO and A549 | Spherical | 5–40 | [169] |
| 2.3 | Achyranthes Aspera Linn Seed | Anticancer | HeLa (Cervical) | Spherical, hexagonal, triangular | 9 | [170] |
| 2.4 | Benincasa hispida | Anticancer | HeLa (Cervical) | Spherical | 23 | [126] |
| 2.5 | Brazilian red propolis | Anticancer | Bladder (T24) and prostate (PC-3) | Rods, triangular, pentagonal, hexagonal | 8–15 | [127] |
| 2.6 | Couroupita guianensis | Anticancer | HL-60 | Cubic | 27 | [171,172] |
| 2.7 | Curcuma wenyujin | Anticancer | A498(renal carcinoma) | Spherical | 200 | [173] |
| 2.8 | Ceiba pentandra (L) | Anticancer | HCT-116 (colon cancer) | Spherical | 20–48 | [135] |
| 2.9 | Corchorus olitorius | Antiproliferative effect | (Breast) MCF-7, (colon) HCT-11, and (hepatocellular) HepG-2 |
Triangular, hexagonal | 37–50 | [174] |
| 2.10 | Diospyros ferrea | Anticancer | HeLa | Diverse | 70–90 | [136] |
| 2.11 | Dioscorea batatas | Cytotoxicity | B16/F10 (melanoma) | Diverse | 19–56 | [137] |
| 2.12 | Dracocephalum kotschyi | Anticancer | K562 and HeLa | Spherical | 11 | [140] |
| 2.13 | Bay cedar | Anticancer | Cervical cancer (HeLa) | Spherical | 20–25 | [143] |
| 2.14 | Hevea brasiliensis | Cytoxicity and genotoxicity | CHO-K1 cells | Spherical, triangular | 50 | [147] |
| 2.15 | Justica wynaadensis | Anticancer | (Lung cancer) A549 | Spherical | 30–50 | [148] |
| 2.16 | Jasminum auriculatum | Anticancer | Cervical cancer (HeLa) | Spherical | 8–37 | [149] |
| 2.17 | Lobila nicotianifolia | Anticancer | (Lung cancer) A459 | Spherical | 80 | [150] |
| 2.18 | Musa paradisiaca (Banana) | Anticancer | (Lung cancer) A459 | Diverse | 300 | [154] |
| 2.19 | Marsdenia tenacissima | Anticancer | (Lung cancer) A459 | Spherical | 50 | [175] |
| 2.20 | Marsilea quadrifolia | Anticancer | (Lung adenocarcinoma) (A549) | Spherical | 10–40 | [176] |
| 2.21 | Mangifera indica (MI) mango peel | Cytotoxicity | African green monkey kidney normal cells (CV-1) and fetal lung fibroblast cells (WI-38) | Round, triangular, irregular | 19–45 | [177] |
| 2.22 | Nerium oleander | Anticancer | MCF-7 (breast cancer) | Spherical | 2–10 | [178] |
| 2.23 | Nepeta deflersiana | Anticancer | (Human cervical) HeLA | Cubic | 33 | [179] |
| 2.24 | Nigella arvensis | Cytotoxicity and catalytic activities | H1299 and MCF-7 | Spherical | 3–37 | [156] |
| 2.25 | Orchid | Anticancer | AMG-13 (breast cancer) | Spherical | 14–50 | [180] |
| 2.26 | Punica granatum | Anticancer | HeLa | Spherical | 5–20 | [157] |
| 2.27 | Korean red ginseng | Anticancer | (cervical), HeLa, Hep2 | Spherical | 3–40 | [181] |
| 2.28 | Padina tetrastromatica | Anticancer | Liver cancer (HepG2) and lung cancer (A549) | Spherical | 8–10 | [182] |
| 2.29 | Scutellaria barbata | Anticancer | Pancreatic (PANC-1) | Spherical | 154 | [183] |
| 2.30 | saffron stigma (crocin) | Anticancer | Human breast cancer cell line (MCF-7) | Spherical | 4–10 | [184] |
| 2.31 | Sargassum swartzii | Anticancer | Human cervical carcinoma (HeLa) | Spherical | 35 | [185] |
| 2.32 | Seaweed | Anticancer | MCF-7 (breast cancer) | Cubic, spherical | 20–50 | [186] |
| 2.33 | Taxus baccata | Anticancer | Breast cells (MCF-7), cervical cells (HeLa) and ovarian cells (Caov-4) | Dispersed | 20 | [187] |
| 2.34 | Wedelia trilobata | Anticancer | HCT 15 (colon cancer) | Spherical, cubic | 10–50 | [188] |
| 2.35 | Piper betle | Cytotoxicity | HeLa and HEK293 | Prism, cubic, octahedron, tetrahedron, dodecahedron, triangular | 15–55 | [189] |
| 3 | Nanoparticles with Antifungal activity | |||||
| 3.1 |
Abelmoschus
esculentus (Okra) |
Antifungal | Crystalline | 62 | [190] | |
| 3.2 | Artemisia vulgaris (Mugwort) | Larvicidal activity against Aedes larvae |
Spherical, triangular, hexagonal | 50–100 | [191] | |
| 3.3 | Brazilian red propolis | Antifungal | Rods, triangular, pentagonal, hexagonal | 8–15 | [127] | |
| 3.4 | Coreopsis lanceolate | Detections of aflatoxins | [129] | |||
| 3.5 | Carthamus tinctorius L | Antifungal | Triangular, spherical | 40–200 | [131] | |
| 3.6 | Caesalpinia pulcherrima | Antifungal | Spherical | 10–50 | [130] | |
| 3.7 | Bay cedar | Antifungal | Spherical | 20–25 | [143] | |
| 3.8 | Helianthus annuus | Antifungal | Polydispersed | 35 | [146] | |
| 3.9 | Nepenthes khasiana | Antifungal | Spherical | 50–80 | [155] | |
| 3.10 | Punica granatum | Antifungal | Spherical | 5–20 | [157] | |
| 3.11 | Pistacia integerrima | Antifungal | Granular | 20–200 | [158] | |
| 3.12 | Rivea hypocrateriformis | Antifungal | Spherical | 10–50 | [161] | |
| 3.13 | Trianthema decandra L | Antifungal | Spherical, hexagonal, cuboidal | 38–80 | [166] | |
| 4 | Nanoparticles with Antioxidant activity/antidiabetic activity | |||||
| 4.1 | Areca catechu | Catalyst, antioxidant | HeLa | Spherical | 13.7 | [123] |
| 4.2 |
Clitoria ternatea (Asian
pigeonwings) |
Antioxidant | Spherical, triangular, hexagonal | 10 | [128] | |
| 4.3 | Couroupita guianensis | Antioxidant | HL-60 | Cubic | 27 | [171,172] |
| 4.4 | Hoveniadulcis | Antioxidant | Spherical | 20 | [145] | |
| 4.5 | Justica wynaadensis | Antidiabetic and anti-inflammatory | (Lung cancer) A549 | Spherical | 30–50 | [148] |
| 4.6 | Nerium oleander | Antioxidant | MCF-7 (breast cancer) | Spherical | 2–10 | [178] |
| 4.7 | Nigella arvensis | Antioxidant, catalytic activities | H1299 and MCF-7 | Spherical | 3–37 | [156] |
It is a known fact that using plants to make gold nanoparticles can result in nanoparticles with distinct biological properties. In a recent study by Mobaraki et al., 2021, using Achillea biebersteinii flower extract, spherical-shaped (8 nm) gold nanoparticles with anticancer properties against human testicular embryonic carcinoma stem cells were synthesized. The nanoparticles demonstrated dose-dependent cell viability against cancer cells by inducing apoptosis, with half inhibitory concentration (IC50) values of 10 g/mL [192]. In another study, Mousavi-Kouhi et al., 2022, synthesized gold nanoparticles from Verbascum speciosum; the green synthesized AuNPs were about 118 ± 72 nm in size and very effective against the hepatocellular carcinoma cell line (HepG2) and pathogenic bacteria [193].
Researchers are increasingly interested in the use of naturally occurring materials in biomedicine, and gum tragacanth (GT) has recently shown great promise as a therapeutic substance in tissue engineering and regenerative medicine. GT is a polysaccharide that can be extracted easily from the stems and branches of various Astragalus species. This anionic polymer is biodegradable, non-allergenic, non-toxic, and non-carcinogenic. GT′s resistance to microbial, heat, and acid degradation has made it a popular material in industrial (e.g., food packaging) and biomedical applications (e.g., drug delivery). GT has been shown to be a useful reagent in the formation and stabilization of metal nanoparticles over time [194,195].
5. Future Prospective
When we use green synthesis to make AuNPs, the process is simple. The reaction occurs in a controlled atmosphere with minimal temperature and pressure changes. Their reduction property determines the answer. A plant-based bioactive molecule that functions as a reducing agent usually produces the quickest reaction. Because of the benefits of employing green synthesis, we need to determine which molecules are feasible and to scale-up the commercialization of gold nanoparticles, as well as conduct the research needed for theranostic applications and disease markers. In addition, research should focus on in vivo investigations so that AuNPs can be used further as a medication or carrier for biomedical applications.
6. Conclusions
Diverse medicinal plants and their parts are employed to synthesize AuNPs, which have the unique virtue of having anticancer, antibacterial, and antifungal properties with theranostic applications. Nanotheranostics is a rapidly growing research field with enormous potential for improving disease diagnosis and treatment. Green nanoparticle synthesis, with its low capital requirements and operating costs, reduced pollution, and improved biocompatibility and stability, is a new and emerging field with advantages over chemical and physical nanoparticle synthesis methods. The number of biomedical applications in this sector is growing every day, with bioimaging, drug delivery, biosensors, and gene delivery among them. We hope that by focusing the readers’ attention on naturally synthesized nanoparticles and their applications, this review will help form a new perspective.
Acknowledgments
The authors are grateful to KLE Technological University, Hubbali, Karnataka, India. The authors sincerely acknowledge the Deanship of Scientific Research, Najran University, Najran, Saudi Arabia, for supporting this research through grant research code NU/RC/MRC/11/1.
Funding
The authors are grateful to the Deanship of Scientific Research, Najran University, Najran, Saudi Arabia, for funding this research through grant research code NU/RC/MRC/11/1.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang T., Chen P., Sun Y., Xing Y., Yang Y., Dong Y., Xu L., Yang Z., Liu D. A New Strategy Improves Assembly Efficiency of DNA Mono-Modified Gold Nanoparticles. Chem. Commun. 2011;47:5774–5776. doi: 10.1039/c1cc11337b. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee P., Bhattacharya R., Bone N., Lee Y.K., Patra C.R., Wang S., Lu L., Secreto C., Banerjee P.C., Yaszemski M.J., et al. Potential Therapeutic Application of Gold Nanoparticles in B-Chronic Lymphocytic Leukemia (BCLL): Enhancing Apoptosis. J. Nanobiotechnology. 2007;5:4. doi: 10.1186/1477-3155-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calzolai L., Franchini F., Gilliland D., Rossi F. Protein–Nanoparticle Interaction: Identification of the Ubiquitin–Gold Nanoparticle Interaction Site. Nano Lett. 2010;10:3101–3105. doi: 10.1021/nl101746v. [DOI] [PubMed] [Google Scholar]
- 4.Jamison J.A., Bryant E.L., Kadali S.B., Wong M.S., Colvin V.L., Matthews K.S., Calabretta M.K. Altering Protein Surface Charge with Chemical Modification Modulates Protein–Gold Nanoparticle Aggregation. J. Nanopart. Res. 2011;13:625–636. doi: 10.1007/s11051-010-0057-5. [DOI] [Google Scholar]
- 5.Uehara N. Polymer-Functionalized Gold Nanoparticles as Versatile Sensing Materials. Anal. Sci. 2010;26:1219–1228. doi: 10.2116/analsci.26.1219. [DOI] [PubMed] [Google Scholar]
- 6.Brown S.D., Nativo P., Smith J.-A., Stirling D., Edwards P.R., Venugopal B., Flint D.J., Plumb J.A., Graham D., Wheate N.J. Gold Nanoparticles for the Improved Anticancer Drug Delivery of the Active Component of Oxaliplatin. J. Am. Chem. Soc. 2010;132:4678–4684. doi: 10.1021/ja908117a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan J.A., Kudgus R.A., Szabolcs A., Dutta S., Wang E., Cao S., Curran G.L., Shah V., Curley S., Mukhopadhyay D., et al. Designing Nanoconjugates to Effectively Target Pancreatic Cancer Cells in Vitro and in Vivo. PLoS ONE. 2011;6:e20347. doi: 10.1371/journal.pone.0020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh P., Kim Y.-J., Zhang D., Yang D.-C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016;34:588–599. doi: 10.1016/j.tibtech.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Barry N.P.E., Sadler P.J. Challenges for Metals in Medicine: How Nanotechnology May Help to Shape the Future. ACS Nano. 2013;7:5654–5659. doi: 10.1021/nn403220e. [DOI] [PubMed] [Google Scholar]
- 10.Bakur A., Niu Y., Kuang H., Chen Q. Synthesis of Gold Nanoparticles Derived from Mannosylerythritol Lipid and Evaluation of Their Bioactivities. AMB Express. 2019;9:62. doi: 10.1186/s13568-019-0785-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar R. Anticancer Activity of Eco-Friendly Au Nanoparticles against Lung and Liver Cancer Cells. J. Genet. Eng. Biotechnol. 2016;14:195–202. doi: 10.1016/j.jgeb.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y., Cui X., Shi F., Deng Y. Nano-Gold Catalysis in Fine Chemical Synthesis. Chem. Rev. 2012;112:2467–2505. doi: 10.1021/cr200260m. [DOI] [PubMed] [Google Scholar]
- 13.Duncan T.V. Applications of Nanotechnology in Food Packaging and Food Safety: Barrier Materials, Antimicrobials and Sensors. J. Colloid Interface Sci. 2011;363:1–24. doi: 10.1016/j.jcis.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dastjerdi R., Montazer M. A Review of the Application of Microbial Nanostructured Materials in the Modification of Textiles Focus on Antimicrobial Properties. Colloids Surf. B Biointerfaces. 2010;79:5–8. doi: 10.1016/j.colsurfb.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 15.Mikhailova E.O. Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application. J. Funct. Biomater. 2021;12:70. doi: 10.3390/jfb12040070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basavegowda N., Idhayadhulla A., Lee Y.R. Tyrosinase Inhibitory Activity of Silver Nanoparticles Treated with Hovenia dulcis Fruit Extract: An in Vitro Study. Mater. Lett. 2014;129:28–30. doi: 10.1016/j.matlet.2014.05.008. [DOI] [Google Scholar]
- 17.Kulkarni N., Muddapur U. Biosynthesis of Metal Nanoparticles: A Review. J. Nanotechnol. 2014;2014:510246. doi: 10.1155/2014/510246. [DOI] [Google Scholar]
- 18.Lim Y.Y., Murtijaya J. Antioxidant Properties of Phyllanthus amorous Extracts as Affected by Different Drying Methods. LWT-Food Sci. Technol. 2007;40:1664–1669. doi: 10.1016/j.lwt.2006.12.013. [DOI] [Google Scholar]
- 19.Sathishkumar M., Sneha K., Won S.W., Cho C.W., Kim S., Yan Y.Y. Cinnamomum Zeylanicum Bark Extract and Powder Mediated Green Synthesis of Nano-Crystalline Ag Particles and Its Bacterial Activity. Colloids Surf. B Biointerfaces. 2009;73:332–338. doi: 10.1016/j.colsurfb.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Safaepaser M., Shahverdi A.R., Shahverdi H.R., Khorramizadeh M.R., Gohari A.R. Green Synthesis of Small Ag Nanoparticles Using Geraniol and Its Cytotoxicity against Fibrosarcomawehi 164. Avicenna J. Med. Biotechnol. 2009;1:111–115. [PMC free article] [PubMed] [Google Scholar]
- 21.Ju-Nam Y., Led J.R. Manufactured Nanoparticles: An Overview of Their Chemistry, Inclination, and Potential Implication. Sci. Total Environ. 2008;400:396–4141. doi: 10.1016/j.scitotenv.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 22.Kim K., Jung B., Kim J., Kim W. Effects of Embedding Non-Absorbing Nanoparticles in Organic Photovoltaics on Power Conversion Efficiency. Sol. Energy Mater. Sol. Cells. 2010;94:1835–1839. doi: 10.1016/j.solmat.2010.05.049. [DOI] [Google Scholar]
- 23.Geoprincy G., Srri B.V., Poonguzhali U., Gandhi N.N., Ranganathan S. A Review on Green Synthesis of Ag Nanoparticles. Asian J. Pharm. Nat. 2013;6:8–12. [Google Scholar]
- 24.Kruis F.E., Fissan H., Rellinghaus B. Sintering and Evaporation Characteristics of Gas-Phase Synthesis of Size-Selected PbS Nanoparticles. Mater. Sci. Eng. B. 2000;69–70:329–334. doi: 10.1016/S0921-5107(99)00298-6. [DOI] [Google Scholar]
- 25.Jung J.H., Oh H.C., Noh H.S., Ji J.H., Kim S.S. Metal Nanoparticle Generation Using a Small Ceramic Heater with a Local Heating Area. J. Aerosol Sci. 2006;37:1662–1670. doi: 10.1016/j.jaerosci.2006.09.002. [DOI] [Google Scholar]
- 26.Iravani S., Korbekandi H., Mirmohammadi S.V., Zolfaghari B. Synthesis of Ag Nanoparticles; Chemical, Physical and Biological Methods. Res. Pharm. Sci. 2014;9:385–406. [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuji T., Iryo K., Watanabe N., Tsuji M. Preparation of Ag Nanoparticles by Laser Ablation in Solution: Influence of Laser Wavelength on Particle Size. Appl. Surf. Sci. 2002;202:80–85. doi: 10.1016/S0169-4332(02)00936-4. [DOI] [Google Scholar]
- 28.Tien D.-C., Tseng K.-H., Liao C.-Y., Huam J.C., Tsung T.T. Discovery of Ionic Ag in Ag Nanoparticles Suspension Fabricated by Arc Discharge Method. J. Alloys Compd. 2008;463:408–411. doi: 10.1016/j.jallcom.2007.09.048. [DOI] [Google Scholar]
- 29.Iqbal N., Iqubal S.M.S., Khan A.A., Mohammed T., Alshabi A.M., Aazam E.S., Rafiquee M.Z.A. Effect of CTABr (Surfactant) on the Kinetics of Formation of Silver Nanoparticles by Amla Extract. J. Mol. Liq. 2021;329:115537. doi: 10.1016/j.molliq.2021.115537. [DOI] [Google Scholar]
- 30.Sardar R., Shumaker-Parry J.S. Spectroscopic and Microscopic Investigation of Gold Nanoparticle Formation: Ligand and Temperature Effects on Rate and Particle Size. J. Am. Chem. Soc. 2011;133:8179–8190. doi: 10.1021/ja107934h. [DOI] [PubMed] [Google Scholar]
- 31.Grzelczak M., Pérez-Juste J., Mulvaney P., Liz-Marzán L.M. Shape Control in Gold Nanoparticle Synthesis. Chem. Soc. Rev. 2008;37:1783–1791. doi: 10.1039/b711490g. [DOI] [PubMed] [Google Scholar]
- 32.Turkevich J., Stevenson P.C., Hillier J. A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss. Faraday Soc. 1951;11:55. doi: 10.1039/df9511100055. [DOI] [Google Scholar]
- 33.Wilton-Ely J.D.E.T. The Surface Functionalisation of Gold Nanoparticles with Metal Complexes. Dalton Trans. 2008:25–29. doi: 10.1039/B714144K. [DOI] [PubMed] [Google Scholar]
- 34.Aslan K., Pérez-Luna V.H. Surface Modification of Colloidal Gold by Chemisorption of Alkanethiols in the Presence of a Nonionic Surfactant. Langmuir. 2002;18:6059–6065. doi: 10.1021/la025795x. [DOI] [Google Scholar]
- 35.Lin S.-Y., Tsai Y.-T., Chen C.-C., Lin C.-M., Chen C.-H. Two-Step Functionalization of Neutral and Positively Charged Thiols onto Citrate-Stabilized Au Nanoparticles. J. Phys. Chem. B. 2004;108:2134–2139. doi: 10.1021/jp036310w. [DOI] [Google Scholar]
- 36.Brust M., Walker M., Bethell D., Schiffrin D.J., Whyman R. Synthesis of Thiol-Derivatized Gold Nanoparticles in a Two-Phase Liquid–Liquid System. J. Chem. Soc. Chem. Commun. 1994:801–802. doi: 10.1039/C39940000801. [DOI] [Google Scholar]
- 37.Hostetler M.J., Wingate J.E., Zhong C.-J., Harris J.E., Vachet R.W., Clark M.R., Londono J.D., Green S.J., Stokes J.J., Wignall G.D., et al. Alkanethiolate Gold Cluster Molecules with Core Diameters from 1.5 to 5.2 Nm: Core and Monolayer Properties as a Function of Core Size. Langmuir. 1998;14:17–30. doi: 10.1021/la970588w. [DOI] [Google Scholar]
- 38.Love J.C., Estroff L.A., Kriebel J.K., Nuzzo R.G., Whitesides G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005;105:1103–1169. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- 39.Noruzi M., Zare D., Khoshnevisan K., Davoodi D. Rapid Green Synthesis of Gold Nanoparticles Using Rosa Hybrida Petal Extract at Room Temperature. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011;79:1461–1465. doi: 10.1016/j.saa.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Thirumurugan A., Jiflin G.J., Rajagomathi G., Tomy N.A., Ramachandran S., Jaiganesh R. Biotechnological synthesis of gold nanoparticles of Azadirachta indica leaf extract. Int. J. Biol. Technol. 2010;1:75–77. [Google Scholar]
- 41.Herizchi R., Abbasi E., Milani M., Akbarzadeh A. Current Methods for Synthesis of Gold Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016;44:596–602. doi: 10.3109/21691401.2014.971807. [DOI] [PubMed] [Google Scholar]
- 42.Mohanpuria P., Rana N.K., Yadav S.K. Biosynthesis of Nanoparticles: Technological Concepts and Future Applications. J. Nanoparticle Res. 2008;10:507–517. doi: 10.1007/s11051-007-9275-x. [DOI] [Google Scholar]
- 43.Singh M., Kalaivani R., Manikandan S., Sangeetha N., Kumaraguru A.K. Facile Green Synthesis of Variable Metallic Gold Nanoparticle Using Padina gymnospora, a Brown Marine Macroalga. Appl. Nanosci. 2013;3:145–151. doi: 10.1007/s13204-012-0115-7. [DOI] [Google Scholar]
- 44.Gardea-Torresdey J.L., Parsons J.G., Gomez E., Peralta-Videa J., Troiani H.E., Santiago P., Yacaman M.J. Formation and Growth of Au Nanoparticles inside Live Alfalfa Plants. Nano Lett. 2002;2:397–401. doi: 10.1021/nl015673+. [DOI] [Google Scholar]
- 45.Tippayawat P., Phromviyo N., Boueroy P., Chompoosor A. Green Synthesis of Silver Nanoparticles in Aloe Vera Plant Extract Prepared by a Hydrothermal Method and Their Synergistic Antibacterial Activity. PeerJ. 2016;4:e2589. doi: 10.7717/peerj.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmed S., Annu, Ikram S., Yudha S.S. Biosynthesis of Gold Nanoparticles: A Green Approach. J. Photochem. Photobiol. B. 2016;161:141–153. doi: 10.1016/j.jphotobiol.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 47.Narayanan K.B., Sakthivel N. Coriander Leaf Mediated Biosynthesis of Gold Nanoparticles. Mater. Lett. 2008;62:4588–4590. doi: 10.1016/j.matlet.2008.08.044. [DOI] [Google Scholar]
- 48.Ankamwar B. Biosynthesis of Gold Nanoparticles (Green-Gold) Using Leaf Extract Of Terminalia catappa. J. Chem. 2010;7:1334–1339. doi: 10.1155/2010/745120. [DOI] [Google Scholar]
- 49.Smitha S.L., Philip D., Gopchandran K.G. Green Synthesis of Gold Nanoparticles Using Cinnamomum zeylanicum Leaf Broth. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009;74:735–739. doi: 10.1016/j.saa.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 50.Parida U.K., Bindhani B.K., Nayak P. Green Synthesis and Characterization of Gold Nanoparticles Using Onion (Allium cepa) Extract. World J. Nano Sci. Eng. 2011;1:93–98. doi: 10.4236/wjnse.2011.14015. [DOI] [Google Scholar]
- 51.Vadlapudi V., Kaladhar D.S.V.G.K. Review: Green Synthesis of Silver and Gold Nanoparticles. Middle-East J. Sci. Res. 2014;19:834–842. [Google Scholar]
- 52.Arunachalam K.D., Annamalai S.K., Hari S. One-Step Green Synthesis and Characterization of Leaf Extract-Mediated Biocompatible Silver and Gold Nanoparticles from Memecylon umbellatum. Int. J. Nanomed. 2013;8:1307–1315. doi: 10.2147/IJN.S36670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aromal S.A., Vidhu V.K., Philip D. Green Synthesis of Well-Dispersed Gold Nanoparticles Using Macrotyloma uniflorum. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012;85:99–104. doi: 10.1016/j.saa.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 54.Kalishwaralal K., Deepak V., Ram Kumar Pandian S., Kottaisamy M., BarathmaniKanth S., Kartikeyan B., Gurunathan S. Biosynthesis of Silver and Gold Nanoparticles Using Brevibacterium casei. Colloids Surf. B Biointerfaces. 2010;77:257–262. doi: 10.1016/j.colsurfb.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Sujitha M.V., Kannan S. Green Synthesis of Gold Nanoparticles Using Citrus Fruits (Citrus limon, Citrus reticulata and Citrus sinensis) Aqueous Extract and Its Characterization. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013;102:15–23. doi: 10.1016/j.saa.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 56.Tamuly C., Hazarika M., Borah S.C., Das M.R., Boruah M.P. In Situ Biosynthesis of Ag, Au and Bimetallic Nanoparticles Using Piper pedicellatum C.DC: Green Chemistry Approach. Colloids Surf. B Biointerfaces. 2013;102:627–634. doi: 10.1016/j.colsurfb.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 57.Kumar K.M., Mandal B.K., Sinha M., Krishnakumar V. Terminalia chebula Mediated Green and Rapid Synthesis of Gold Nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012;86:490–494. doi: 10.1016/j.saa.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 58.Elavazhagan T., Arunachalam K.D. Memecylon edule Leaf Extract Mediated Green Synthesis of Silver and Gold Nanoparticles. Int. J. Nanomed. 2011;6:1265–1278. doi: 10.2147/IJN.S18347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Das R.K., Gogoi N., Bora U. Green Synthesis of Gold Nanoparticles Using Nyctanthes arbortristis Flower Extract. Bioprocess Biosyst. Eng. 2011;34:615–619. doi: 10.1007/s00449-010-0510-y. [DOI] [PubMed] [Google Scholar]
- 60.Philip D., Unni C., Aromal S.A., Vidhu V.K. Murraya koenigii Leaf-Assisted Rapid Green Synthesis of Silver and Gold Nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011;78:899–904. doi: 10.1016/j.saa.2010.12.060. [DOI] [PubMed] [Google Scholar]
- 61.Santhoshkumar J., Rajeshkumar S., Venkat Kumar S. Phyto-Assisted Synthesis, Characterization and Applications of Gold Nanoparticles—A Review. Biochem. Biophys. Rep. 2017;11:46–57. doi: 10.1016/j.bbrep.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bankar A., Joshi B., Kumar A.R., Zinjarde S. Banana Peel Extract Mediated Synthesis of Gold Nanoparticles. Colloids Surf. B Biointerfaces. 2010;80:45–50. doi: 10.1016/j.colsurfb.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 63.Vinod V.T.P., Saravanan P., Sreedhar B., Devi D.K., Sashidhar R.B. A Facile Synthesis and Characterization of Ag, Au and Pt Nanoparticles Using a Natural Hydrocolloid Gum Kondagogu (Cochlospermum gossypium) Colloids Surf. B Biointerfaces. 2011;83:291–298. doi: 10.1016/j.colsurfb.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 64.Kumar K.P., Paul W., Sharma C.P. Green Synthesis of Gold Nanoparticles with Zingiber Officinale Extract: Characterization and Blood Compatibility. Process Biochem. 2011;46:2007–2013. doi: 10.1016/j.procbio.2011.07.011. [DOI] [Google Scholar]
- 65.Shrikanth V.M., Janardhan B., Dhananjaya B.L., Muddapura U.M., More S.S. Antimicrobial And Antioxidant Activity Of Methanolic Root Extract Of Tabernaemontana alternifolia L. Int. J. Pharm. Pharm. Sci. 2015;7:66–69. [Google Scholar]
- 66.Majumdar R., Bag B.G., Ghosh P. Mimussops Elengi Bark Extract Mediated Green Synthesis of Au Nanoparticles and Study of Its Catalytic Activity. Appl. Nanosci. 2016;6:521–528. doi: 10.1007/s13204-015-0454-2. [DOI] [Google Scholar]
- 67.Shankar S.S., Rai A., Ahamed A., Sastry M. Rapid Synthesis of Au, Ag and Bimetallic Au Core Ag Shell Nanoparticles Using Neem (Azadictira indica) Leaf Broth. J. Colloid Interface. 2004;275:496–502. doi: 10.1016/j.jcis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 68.Bakar A., Ismail N.H.H., Abu Bakar J. Synthesis and Characterization of Ag Nanoparticles in Natural Rubber. Matter. Chem. Phys. 2007;104:276–283. doi: 10.1016/j.matchemphys.2007.03.015. [DOI] [Google Scholar]
- 69.Chandan S.P., Chaudhary M., Pasricha R., Ahmad A., Sastry M. Synthesis of Au Nano Triangles and Ag Nanoparticles Using Aloevera Plant Extract. Biotechnol. Prog. 2006;22:577–583. doi: 10.1021/bp0501423. [DOI] [PubMed] [Google Scholar]
- 70.Shankar S.S., Rai A., Ahamed A., Sastry M. Controlling the Optical Properties of Lemongrass Extract Synthesized Au Nano Triangles and Potential Application Infrared-Absorbing Optical Coatings. Chem. Mater. 2005;17:566–572. doi: 10.1021/cm048292g. [DOI] [Google Scholar]
- 71.Kumar V., Singh S., Srivastava B., Bhadouria R., Singh R. Green Synthesis of Silver Nanoparticles Using Leaf Extract of Holoptelea integrifolia and Preliminary Investigation of Its Antioxidant, Anti-Inflammatory, Antidiabetic and Antibacterial Activities. J. Environ. Chem. Eng. 2019;7:103094. doi: 10.1016/j.jece.2019.103094. [DOI] [Google Scholar]
- 72.Vinosha M., Palanisamy S., Muthukrishnan R., Selvam S., Kannapiran E., You S., Prabhu N.M. Biogenic Synthesis of Gold Nanoparticles from Halymenia dilatata for Pharmaceutical Applications: Antioxidant, Anti-Cancer and Antibacterial Activities. Process Biochem. 2019;85:219–229. doi: 10.1016/j.procbio.2019.07.013. [DOI] [Google Scholar]
- 73.Barai A.C., Paul K., Dey A., Manna S., Roy S., Bag B.G., Mukhopadhyay C. Green Synthesis of Nerium Oleander-Conjugated Gold Nanoparticles and Study of Its in Vitro Anticancer Activity on MCF-7 Cell Lines and Catalytic Activity. Nano Converg. 2018;5:10. doi: 10.1186/s40580-018-0142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patil M.P., Bayaraa E., Subedi P., Piad L.L.A., Tarte N.H., Kim G.D. Biogenic Synthesis, Characterization of Gold Nanoparticles Using Lonicera japonica and Their Anticancer Activity on HeLa Cells. J. Drug Deliv. Sci. Technol. 2019;51:83–90. doi: 10.1016/j.jddst.2019.02.021. [DOI] [Google Scholar]
- 75.Castro-Aceituno V., Abbai R., Moon S.S., Ahn S., Mathiyalagan R., Kim Y.-J., Kim Y.-J., Yang D.C. Pleuropterus Multiflorus (Hasuo) Mediated Straightforward Eco-Friendly Synthesis of Silver, Gold Nanoparticles and Evaluation of Their Anticancer Activity on A549 Lung Cancer Cell Line. Biomed. Pharmacother. 2017;93:995–1003. doi: 10.1016/j.biopha.2017.07.040. [DOI] [PubMed] [Google Scholar]
- 76.Arulkumar S., Sabesan M. Biosynthesis and Characterization of Au Nanoparticles Using Anti Parkinsonian Drug Mucuna purines Plant Extract. Int. J. Res. Pharm. Sci. 2010;1:417–420. [Google Scholar]
- 77.Dash S.S., Majumdar R., Sikder A.K., Bag B.G., Patra B.K. Saraca indica Bark Extract Mediated Green Synthesis of Polyshaped Gold Nanoparticles and Its Application in Catalytic Reduction. Appl. Nanosci. 2014;4:485–490. doi: 10.1007/s13204-013-0223-z. [DOI] [Google Scholar]
- 78.Wang C., Mathiyalagan R., Kim Y.J., Castro-Aceituno V., Singh P., Ahn S., Wang D., Yang D.C. Rapid Green Synthesis of Silver and Gold Nanoparticles Using Dendropanax morbifera Leaf Extract and Their Anti-Cancer Activities. Int. J. Nanomed. 2016;10:3691–3701. doi: 10.2147/IJN.S97181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Geethalakshmi R., Sarada D.V.L. Gold and Silver Nanoparticles from Trianthema decandra: Synthesis, Characterization, and Antimicrobial Properties. Int. J. Nanomed. 2012;7:5375–5384. doi: 10.2147/IJN.S36516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Balasubramani G., Ramkumar R., Krishnaveni N., Pazhanimuthu A., Natarajan T., Sowmiya R., Perumal P. Structural Characterization, Antioxidant and Anticancer Properties of Gold Nanoparticles Synthesized from Leaf Extract(Decoction)of Antigonon leptopus Hook. & Arn. J. Trace Elements Med. Biol. 2015;30:83–89. doi: 10.1016/j.jtemb.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 81.Raghunandan D., Ravishankar B., Sharanbasava G., Mahesh D.B., Harsoor V., Yalagatti M.S., Bhagawanraju M., Venkataraman A. Anti-Cancer Studies of Noble Metal Nanoparticles Synthesized Using Different Plant Extracts. Cancer Nanotechnol. 2011;2:57–65. doi: 10.1007/s12645-011-0014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Castillo-Henríquez L., Alfaro-Aguilar K., Ugalde-Álvarez J., Vega-Fernández L., Montes de Oca-Vásquez G., Vega-Baudrit J.R. Green Synthesis of Gold and Silver Nanoparticles from Plant Extracts and Their Possible Applications as Antimicrobial Agents in the Agricultural Area. Nanomaterials. 2020;10:1763. doi: 10.3390/nano10091763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Balwe S.G., Rokade A.A., Park S.S., Jeong Y.T. Green Synthesis and Characterization of Supported Gold Nanoparticles (Au@PS) from Schisandra Chinensis Fruit Extract: An Efficient and Reusable Catalyst for the Synthesis of Chromeno[2,3-d]Pyrimidin-2-Yl)Phenol Derivatives under Solvent-Free Conditions. Catal. Commun. 2019;128:105703. doi: 10.1016/j.catcom.2019.05.010. [DOI] [Google Scholar]
- 84.Singh C., Baboota R.K., Naik P.K., Singh H. Biocompatible Synthesis of Silver and Gold Nanoparticles Using Leaf Extract of Dalbergia sissoo. Adv. Mater. Lett. 2012;3:279–285. doi: 10.5185/amlett.2011.10312. [DOI] [Google Scholar]
- 85.Sermakkani M., Pandian T. Biological Synthesis of Ag Nanoparticles Using Medicinal Plant (Cassia italica) Leaves. Int. J. Curr. Res. 2012;4:53–58. [Google Scholar]
- 86.Konwar Boruah S., Kumar Boruah P., Sarma P., Medhi C., Medhi O.K. Green Synthesis of Gold Nanoparticles Using Camellia sinensis and Kinetics of the Reaction. Adv. Mater. Lett. 2012;3:481–486. doi: 10.5185/amlett.2012.icnano.103. [DOI] [Google Scholar]
- 87.Ahmad M.Z., Akhter S., Rahman Z., Akhter S., Anwar M., Mallik N., Ahmad F.J. Nanometric Gold in Cancer Nanotechnology: Current Status and Future Prospect. J. Pharm. Pharmacol. 2013;65:634–651. doi: 10.1111/jphp.12017. [DOI] [PubMed] [Google Scholar]
- 88.Shiva M.P. Inventory of Forestry Resources for Sustainable Management and Biodiversity Conservation. Indus Publishing; New Delhi, India: 1996. [Google Scholar]
- 89.Cowan M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/CMR.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Adesokan A.A., Yakuba M.T., Owoyele B.V., Akanji M.A., Soladoge A., Lawal O. Effect of Administration of Aqueous and Ethanolic Extracts of Enantia cloranthasteen Bark on Brewer’s Yeast Induced Pepsis in Rats. Afr. J. Biochem. Res. 2008;2:165–169. [Google Scholar]
- 91.Owolabi M.A., Coker H.A., Jaja S.I. Flavonoid Metabolites in Urine after Oral Administration of the Aqueous Extract of Persea americana to Rats. J. Nat. Med. 2007;61:200–204. doi: 10.1007/s11418-006-0122-7. [DOI] [Google Scholar]
- 92.Subramanian V., Gautam V., Raman R., Prahalathan S., Ashish K. The Case of Selected Indian Health Care System. Export-Import Bank of India; Mumbai, India: 2003. [Google Scholar]
- 93.John D., Britto A., Sujin M., Dhurmar M.R. Ethnomedicinal Wisdom of the Manavalakarchi People in Kawgkumari District, Tamilnadu. Int. J. Biol. Technol. 2010;1:25–30. [Google Scholar]
- 94.Ullah S., Khan M.R., Alishah N., Shah S., Majid M. Ethno Medical Plant Use-Value in the Lakki Marwat District of Pakistan. J. Ethnopharmacol. 2014;158:412–422. doi: 10.1016/j.jep.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 95.Yuan H., Ma Q., Ye L., Piao G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules. 2016;21:559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singh S.P. Himalayan Forest and Ecosystem Services: Incorporating in National Accounting. Central Himalayan Environment Association (CHEA); Nainital, Uttarakhand, India: 2007. [Google Scholar]
- 97.Jain S.K. Medicinal Plants. National Book Trust; Delhi, India: 1968. pp. 1–216. [Google Scholar]
- 98.Rajasekharreddy P., Rani P.U., Sreedhar B. Qualitative Assessment of Silver and Goldnanoparticle Synthesis in Various Plants: A Photobiological Approach. J. Nanopart. Res. 2010;12:1711–1721. doi: 10.1007/s11051-010-9894-5. [DOI] [Google Scholar]
- 99.Huang J., Li Q., Sun D., Lu Y., Su Y., Yang X., Wang H., Wang Y., Shao W., He N., et al. Biosynthesis of Silver and Gold Nanoparticles by Novel Sundried Cinnamomum camphora Leaf. Nanotechnology. 2007;18:105104. doi: 10.1088/0957-4484/18/10/105104. [DOI] [Google Scholar]
- 100.Vilchis-Nestor A.R., Sánchez-Mendieta V., Camacho-López M.A., Gómez-Espinosa R.M., Camacho-López M.A., Arenas-Alatorre J.A. Solvent Less Synthesis and Optical Properties of Au and Ag Nanoparticles Using Camellia sinensis Extract. Mater. Lett. 2008;62:3103–3105. doi: 10.1016/j.matlet.2008.01.138. [DOI] [Google Scholar]
- 101.Noruzi M., Zare D., Davoodi D. A Rapid Biosynthesis Route for the Preparation of Gold Nanoparticles by Aqueous Extract of Cypress Leaves at Room Temperature. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012;94:84–88. doi: 10.1016/j.saa.2012.03.041. [DOI] [PubMed] [Google Scholar]
- 102.Jiang X., Sun D., Zhang G., He N., Liu H., Huang J., Odoom-Wubah T., Li Q. Investigation of Active Biomolecules Involved in the Nucleation and Growth of Gold Nanoparticles by Artocarpus heterophyllus Lam Leaf Extract. J. Nanopart. Res. 2013;15:1741. doi: 10.1007/s11051-013-1741-z. [DOI] [Google Scholar]
- 103.Yasmin A., Ramesh K., Rajeshkumar S. Optimization and Stabilization of Gold Nanoparticles by Using Herbal Plant Extract with Microwave Heating. Nano Converg. 2014;1:12. doi: 10.1186/s40580-014-0012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu J., Xu D., Guan H.N., Wang C., Huang L.K., Chi D.F. Facile One-Step Green Synthesis of Gold Nanoparticles Using Citrus maxima Aqueous Extracts and Its Catalytic Activity. Mater. Lett. 2016;166:110–112. doi: 10.1016/j.matlet.2015.12.031. [DOI] [Google Scholar]
- 105.Aromal S.A., Philip D. Green Synthesis of Gold Nanoparticles Using Trigonella foenumgraecum and Its Size Dependent Catalytic Activity. Spectrochim. Acta Part A Mol. Biomol. 2012;97:1–5. doi: 10.1016/j.saa.2012.05.083. [DOI] [PubMed] [Google Scholar]
- 106.Annamalai A., Babu S.T., Jose N.A., Sudha D., Lyza C.V. Biosynthesis and characterization of silver and gold nanoparticles using aqueous leaf extraction of Phyllanthus amarus Schum & Thonn. World Appl. Sci. J. 2011;13:1833–1840. [Google Scholar]
- 107.Andrei A.B., Hassan Y., Serban F. FTIR Spectrophotometric Methods Used for Antioxidant Activity Assay in Medicinal Plants. Appl. Spectrosc. Rev. 2012;47:245–255. [Google Scholar]
- 108.Bhuvanasree S.R., Harini D., Rajaram A., Rajaram R. Rapidsyn-thesis of gold nanoparticles with Cissus quadrangularis extract usingmicrowave ir-radiation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013;106:190–196. doi: 10.1016/j.saa.2012.12.076. [DOI] [PubMed] [Google Scholar]
- 109.Ghodake G.S., Deshpande N.G., Lee Y.P., Jin E.S. Pear Fruit Extract-Assisted Room-Temperature Biosynthesis of Gold Nanoplates. Colloids Surf. B Biointerfaces. 2010;75:584–589. doi: 10.1016/j.colsurfb.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 110.Pasca R.-D., Mocanu A., Cobzac S.-C., Petean I., Horovitz O., Tomoaia-Cotisel M. Biogenic Syntheses of Gold Nanoparticles Using Plant Extracts. Part. Sci. Technol. 2014;32:131–137. doi: 10.1080/02726351.2013.839589. [DOI] [Google Scholar]
- 111.Jun S.H., Kim H.-S., Koo Y.K., Park Y., Kim J., Cho S., Park Y. Root Extracts of Polygala tenuifolia for the Green Synthesis of Gold Nanoparticles. J. Nanosci. Nanotechnol. 2014;14:6202–6208. doi: 10.1166/jnn.2014.8881. [DOI] [PubMed] [Google Scholar]
- 112.Putnam C.D., Hammel M., Hura G.L., Tainer J.A. X-Ray Solution Scattering (SAXS.) Combined with Crystallography and Computation: Defining Accurate Macromolecular Structures, Conformations and Assemblies in Solution. Q. Rev. Biophys. 2007;40:191–285. doi: 10.1017/S0033583507004635. [DOI] [PubMed] [Google Scholar]
- 113.Dubey S.P., Lahtinen M., Sillanpää M. Tansy Fruit Mediated Greener Synthesis of Silver and Gold Nanoparticles. Process Biochem. 2010;45:1065–1071. doi: 10.1016/j.procbio.2010.03.024. [DOI] [Google Scholar]
- 114.Geng G., Chen P., Guan B., Liu Y., Yang C., Wang N., Liu M. Sheetlike Gold Nanostructures/Graphene Oxide Composites via a One-Pot Green Fabrication Protocol and Their Interesting Two-Stage Catalytic Behaviors. RSC Adv. 2017;7:51838–51846. doi: 10.1039/C7RA11188F. [DOI] [Google Scholar]
- 115.Das P., Ghosal K., Jana N.K., Mukherjee A., Basak P. Green Synthesis and Characterization of Silver Nanoparticles Using Belladonna Mother Tincture and Its Efficacy as a Potential Antibacterial and Anti-Inflammatory Agent. Mater. Chem. Phys. 2019;228:310–317. doi: 10.1016/j.matchemphys.2019.02.064. [DOI] [Google Scholar]
- 116.Sozer N., Kokini J.L. Nanotechnology and Its Applications in the Food Sector. Trends Biotechnol. 2009;27:82–89. doi: 10.1016/j.tibtech.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 117.Cai W., Gao T., Hong H., Sun J. Applications of Gold Nanoparticles in Cancer Nanotechnology. Nanotechnol. Sci. Appl. 2008;1:17–32. doi: 10.2147/NSA.S3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tang S., Hewlett I. Nanoparticle-Based Immunoassays for Sensitive and Early Detection of HIV-1 Capsid (P24) Antigen. J. Infect. Dis. 2010;201:S59–S64. doi: 10.1086/650386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ayan K., Barui R., Kotcherlakota C.-H.R. Medicinal Applications of Metal Nanoparticles Synthesis and Applications in Pharmaceutical Sciences. John Wiley & Sons; Hoboken, NJ, USA: 2018. [Google Scholar]
- 120.Riss T.L., Moravec R.A., O’Brien M.A., Hawkins E.M., Niles A. Handbook of Assay Development in Drug Discovery. CRC Press; Boca Raton, FL, USA: 2006. Homogeneous multiwell assays for measuring cell viability, cytotoxicity, and apoptosis; pp. 385–406. [Google Scholar]
- 121.Nakkala J.R., Mata R., Bhagat E., Sadras S.R. Green Synthesis Ofsilver and Gold Nanoparticles from Gymnema Sylvestre Leafextract: Study of Antioxidant and Anti-Cancer Activities. J. Nanopart. Res. 2015;17:151. doi: 10.1007/s11051-015-2957-x. [DOI] [Google Scholar]
- 122.Mukherjee S., Ghosh S., Das D.K. Gold-Conjugated Green Teananoparticles for Enhanced Anti-Tumor Activities and Hepatoprotection-Synthesis, Characterization and in Vitro Evaluation. J. Nutr. Biochem. 2015;26:1283–1297. doi: 10.1016/j.jnutbio.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 123.Rajan A., Vilas V., Philip D. Studies on Catalytic, Antioxidant, Antibacterial and Anti-Cancer Activities of Biogenic Gold Nanoparticles. J. Mol. Liq. 2015;212:331–339. doi: 10.1016/j.molliq.2015.09.013. [DOI] [Google Scholar]
- 124.Ganesan R., Prabu H.G. Synthesis of Gold NanoparticlesUsing Herbal Acorus Calamus Rhizome Extract And Coating on Cotton Fabric for Antibacterial and UV Blocking Applications. Arab. J. Chem. 2015;12:2166–2174. doi: 10.1016/j.arabjc.2014.12.017. [DOI] [Google Scholar]
- 125.Bindhu M., Umadevi M. Antibacterial Activities of Green Synthesized Gold Nanoparticles. Mater. Lett. 2014;120:122–125. doi: 10.1016/j.matlet.2014.01.108. [DOI] [Google Scholar]
- 126.Al Saqr A., Khafagy E.-S., Alalaiwe A., Aldawsari M.F., Alshahrani S.M., Anwer M.K., Khan S., Lila A.S.A., Arab H.H., Hegazy W.A.H. Synthesis of Gold Nanoparticles by Using Green Machinery: Characterization and in Vitro Toxicity. Nanomaterials. 2021;11:808. doi: 10.3390/nano11030808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Botteon C.E.A., Silva L.B., Ccana-Ccapatinta G.V. Biosynthesis and Characterization of Gold Nanoparticles Using Brazilian Red Propolis and Evaluation of Its Antimicrobial and Anti-Cancer Activities. Sci. Rep. 2021;11:1974. doi: 10.1038/s41598-021-81281-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vanaraj S., Jabastin J., Sathiskumar S., Preethi K. Production and Characterization of Bio-AuNPs to Induce Synergistic Effect against Multidrug Resistant Bacterial Biofilm. J. Cluster Sci. 2017;28:227–244. doi: 10.1007/s10876-016-1081-0. [DOI] [Google Scholar]
- 129.Abhijith K.S., Thakur M.S. Application of Green Synthesis of Gold Nanoparticles for Sensitive Detection of Aflatoxin B1 Based on Metal Enhanced Fluorescence. Anal. Methods. 2012;4:4250–4256. doi: 10.1039/c2ay25979f. [DOI] [Google Scholar]
- 130.Nagaraj B., Divya T., Malakar B., Krishnamurthy N., Dinesh R., Negrila C., Ciobanu C., Iconaru S. Phytosynthesis of Gold Nanoparticles Using Caesalpinia pulcherrima (Peacock Flower) Flower Extract And Evaluation of Their Antimicrobial Activities. Dig. J. Nanomater. Biostructures. 2012;7:899–905. [Google Scholar]
- 131.Nagaraj B., Malakar B., Divya T., Krishnamurthy N., Liny P., Dinesh R., Iconaru S., Ciobanu C. Synthesis of Plant Mediated Gold Nanoparticles Using Flower Extracts of Carthamus tinctorius L. (Safflower) and Evaluation Of Their Biological Activities. Dig. J. Nanomater. Biostructures. 2012;7:1289–1296. [Google Scholar]
- 132.Ke Y., Al Aboody M.S., Alturaiki W., Alsagaby S.A., Alfaiz F.A., Veeraraghavan V.P., Mickymaray S. Photosynthesized Gold Nanoparticles from Catharanthus roseus Induces Caspase-Mediated Apoptosis in Cervical Cancer Cells (HeLa) Artif. Cells Nanomed. Biotechnol. 2019;47:1938–1946. doi: 10.1080/21691401.2019.1614017. [DOI] [PubMed] [Google Scholar]
- 133.Muthukumar T., Sambandam B., Aravinthan A., Sastry T.P., Kim J.-H. Green Synthesis of Gold Nanoparticlesand Their Enhanced Synergistic Antitumor Activity Using HepG2 and MCF7 Cells and Its Antibacterial Effects. Process Biochem. 2016;51:384–391. doi: 10.1016/j.procbio.2015.12.017. [DOI] [Google Scholar]
- 134.Naraginti S., Sivakumar A. Eco-Friendly Synthesis of Silver and Gold Nanoparticles with Enhanced Bactericidal Activity and Study of Silver Catalyzed Reduction of 4-Nitrophenol. Spectrochim. Acta Part A Mol. Biomol. 2014;128:357–362. doi: 10.1016/j.saa.2014.02.083. [DOI] [PubMed] [Google Scholar]
- 135.Brian M.O., Selvi S. Biosynthesis and characterization of gold nanoparticles from Ceiba pentandra (L.) Gaertn bark and evaluation of its antibacterial and anticancer activity. Int. J. Res. Pharm. Sci. 2020;35:5643–5650. [Google Scholar]
- 136.Ramesh V., Armash A. Green Synthesis of Gold Nanoparticles Against Pathogens and Cancer Cells. IJPR. 2015;10:250–256. [Google Scholar]
- 137.Sreekanth T.V.M., Nagajyothi P.C., Supraja N., Prasad T.N.V.K.V. Evaluation of the Antimicrobial Activity and Cytotoxicity of Phytogenic Gold Nanoparticles. Appl. Nanosci. 2015;5:595–602. doi: 10.1007/s13204-014-0354-x. [DOI] [Google Scholar]
- 138.Khan A.U., Yuan Q., Wei Y., Khan G.M., Khan Z.U.H., Khan S., Ali F., Tahir K., Ahmad A., Khan F.U. Photocatalytic and Antibacterial Response of Biosynthesized Gold Nanoparticles. J. Photochem. Photobiol. B Biol. 2016;162:273–277. doi: 10.1016/j.jphotobiol.2016.06.055. [DOI] [PubMed] [Google Scholar]
- 139.Dorosti N., Jamshidi F. Plant-Mediated Gold Nanoparticles by Dracocephalum kotschyi as Anticholinesterase Agent: Synthesis, Characterisation, and Evaluation of Anticancer and Antibacterial Activity. J. Appl. Biomed. 2016;14:235–245. doi: 10.1016/j.jab.2016.03.001. [DOI] [Google Scholar]
- 140.Annamalai A., Christina V.L.P., Sudha D., Kalpana M., Lakshmi P.T.V. Green Synthesis, Characterization and Antimicrobial Activity of Au NPs Using Euphorbia hirta L. Leaf Extract. Colloids Surf. B Biointerfaces. 2013;108:60–65. doi: 10.1016/j.colsurfb.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 141.Gopinath K., Kumaraguru S., Bhakyaraj K., Mohan S., Venkatesh K.S., Esakkirajan M., Kaleeswarran P., Alharbi N.S., Kadaikunnan S., Govindarajan M. Green Synthesis of Silver, Gold and Silver/Gold Bimetallic Nanoparticles Using the Gloriosa superba Leaf Extract and Their Antibacterial and Antibiofilm Activities. Microb. Pathog. 2016;101:1–11. doi: 10.1016/j.micpath.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 142.Abdel-Raouf N., Al-Enazi N.M., Ibraheem I.B. Green Biosynthesis of Gold Nanoparticles Using Galaxaura elongata and Characterization of Their Antibacterial Activity. Arab. J. Chem. 2013;10:S3029–S3039. doi: 10.1016/j.arabjc.2013.11.044. [DOI] [Google Scholar]
- 143.Karthika V., Arumugam A., Gopinath K., Kaleeswarran P., Govindarajan M., Alharbi N.S., Kadaikunnan S., Khaled J.M., Benelli G. Guazuma ulmifolia Bark-Synthesized Ag, Au and Ag/Au Alloy Nanoparticles: Photocatalytic Potential, DNA/Protein Interactions, Anticancer Activity and Toxicity against 14 Species of Microbial Pathogens. J. Photochem. Photobiol. B Biol. 2017;167:189–199. doi: 10.1016/j.jphotobiol.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 144.Bindhu M., Rekha P.V., Umamaheswari T., Umadevi M. Antibacterial Activities of Hibiscus cannabinus Stemassisted Silver and Gold Nanoparticles. Mater. Lett. 2014;131:194–197. doi: 10.1016/j.matlet.2014.05.172. [DOI] [Google Scholar]
- 145.Basavegowda N., Idhayadhulla A., Lee Y.R. Phyto-Synthesis of Gold Nanoparticles Using Fruit Extract of Hovenia dulcis and Their Biological Activities. Ind. Crops Prod. 2014;52:745–751. doi: 10.1016/j.indcrop.2013.12.006. [DOI] [Google Scholar]
- 146.Liny P., Divya T., Barasa M., Nagaraj B., Krishnamurthy N., Dinesh R. Preparation of Gold Nanoparticles from Helianthus annuus (Sun Flower) Flowers and Evaluation of Their Antimicrobial Activities. Int. J. Pharma Bio Sci. 2012;3:439–446. [Google Scholar]
- 147.Santos N.M., Gomes A.S., Cavalcante D.G., Santos L.F., Teixeira S.R., Cabrera F.C., Job A.E. Green Synthesis of Colloidal Gold Nanoparticles Using Latex from Hevea brasiliensis and Evaluation of Their in Vitro Cytotoxicity and Genotoxicity. IET Nanobiotechnol. 2019;13:307–315. doi: 10.1049/iet-nbt.2018.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lava M.B., Muddapur U.M., Basavegowda N., More S.S., More V.S. Characterization, anticancer, antibacterial, anti-diabetic and anti-inflammatory activities of green synthesized silver nanoparticles using Justica wynaadensis leaves extract. Mater. Today Proc. 2021;46:5942–5947. doi: 10.1016/j.matpr.2020.10.048. [DOI] [Google Scholar]
- 149.Balasubramanian S., Kala S.M.J., Pushparaj T.L. Biogenic Synthesis of Gold Nanoparticles Using Jasminum auriculatum Leaf Extract and Their Catalytic, Antimicrobial and Anticancer Activities. J. Drug Deliv. Sci. Technol. 2020;57:101620. doi: 10.1016/j.jddst.2020.101620. [DOI] [Google Scholar]
- 150.Lava M.B., Muddapur U.M., Nagaraj B. Synthesis and Characterization of Gold Nanoparticles from Lobelia nicotianifolia Leaf Extract and Its Biological Activities. Adv. Mater. Lett. 2020;11:1–4. doi: 10.5185/amlett.2020.031491. [DOI] [Google Scholar]
- 151.Poojary M.M., Passamonti P., Adhikari A.V. Green Synthesis of Silver and Gold Nanoparticles Using Root Bark Extract of Mammea suriga: Characterization, Process Optimization, and Their Antibacterial Activity. BioNanoScience. 2016;6:110–120. doi: 10.1007/s12668-016-0199-8. [DOI] [Google Scholar]
- 152.Mubarakali D., Thajuddin N., Jeganathan K., Gunasekaran M. Plant Extract Mediated Synthesis of Silver and Gold Nanoparticles and Its Antibacterial Activity Against Clinically Isolated Pathogens. Colloids Surf. B Biointerfaces. 2011;85:360–365. doi: 10.1016/j.colsurfb.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 153.Ahmad A., Syed F., Imran M., Khan A.U., Tahir K., Khan Z.U.H., Yuan Q. Phytosynthesis and AntileishmanialActivity of Gold Nanoparticles by Maytenus Royleanus. J. Food Biochem. 2015;40:420–427. doi: 10.1111/jfbc.12232. [DOI] [Google Scholar]
- 154.Vijayakumar S., Vaseeharan B., Malaikozhundan B., Gopi N., Ekambaram P., Pachaiappan R., Velusamy P., Murugan K., Benelli G., Suresh Kumar R., et al. Therapeutic Effects of Gold Nanoparticles Synthesized Using Musa paradisiaca Peel Extract against Multiple Antibiotic Resistant Enterococcus faecalis Biofilms and Human Lung Cancer Cells (A549) Microb. Pathog. 2017;102:173–183. doi: 10.1016/j.micpath.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 155.Bhau B.S., Ghosh S., Puri S., Borah B., Sarmah D.K., Khan R. Green Synthesis of Gold Nanoparticles from the Leaf Extract of Nepenthes khasiana and Antimicrobial Assay. Adv. Mater. Lett. 2015;6:55–58. doi: 10.5185/amlett.2015.5609. [DOI] [Google Scholar]
- 156.Chahardoli A., Karimi N., Sadeghi F., Fattahi A. Green Approach for Synthesis of Gold Nanoparticles from Nigella arvensis Leaf Extract and Evaluation of Their Antibacterial, Antioxidant, Cytotoxicity and Catalytic Activities. Artif. Cells Nanomed. Biotechnol. 2018;46:579–588. doi: 10.1080/21691401.2017.1332634. [DOI] [PubMed] [Google Scholar]
- 157.Lokina S., Suresh R., Giribabu K., Stephen A., Lakshmi Sundaram R., Narayanan V. Spectroscopic Investigations, Antimicrobial, and Cytotoxic Activity of Green Synthesized Gold Nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014;129:484–490. doi: 10.1016/j.saa.2014.03.100. [DOI] [PubMed] [Google Scholar]
- 158.Islam N.U., Jalil K., Shahid M., Muhammad N., Rauf A. Pistacia integerrima Gall Extract Mediated Green Synthesis of Gold Nanoparticles and Their Biological Activities. Arab. J. Chem. 2019;12:2310–2319. doi: 10.1016/j.arabjc.2015.02.014. [DOI] [Google Scholar]
- 159.Mata R., Bhaskaran A., Sadras S.R. Green-Synthesized Gold Nanoparticles from Plumeria alba Flower Extract to Augment Catalytic Degradation of Organic Dyes and Inhibit Bacterial Growth. Particuology. 2016;24:78–86. doi: 10.1016/j.partic.2014.12.014. [DOI] [Google Scholar]
- 160.Anbu P., Gopinath S.C., Jayanthi S. Synthesis of Gold Nanoparticles Using Platycodon grandiflorum Extract and Its Antipathogenic Activity under Optimal Conditions. Nanomater. Nanotechnol. 2020;10:184798042096169. doi: 10.1177/1847980420961697. [DOI] [Google Scholar]
- 161.Godipurge S.S., Yallappa S., Biradar N.J., Biradar J.S., Dhananjaya B.L., Hegde G., Jagadish K., Hegde G. A Facile and Green Strategy for the Synthesis of Au, Ag and Au–Ag Alloy Nanoparticles Using Aerial Parts of R. hypocrateriformis Extract and Their Biological Evaluation. Enzyme Microb. Technol. 2016;95:174–184. doi: 10.1016/j.enzmictec.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 162.Muthuvel A., Adavallan K., Balamurugan K., Krishnakumar N. Biosynthesis of Gold Nanoparticles Using Solanum nigrum Leaf Extract and Screening Their Free Radical Scavenging and Antibacterial Properties. Biomed. Prev. Nutr. 2014;4:325–332. doi: 10.1016/j.bionut.2014.03.004. [DOI] [Google Scholar]
- 163.Ahmed K.B.A., Subramanian S., Sivasubramanian A., Veerappan G., Veerappan A. Preparation of Gold Nanoparticles Using Salicornia brachiata Plant Extract and Evaluation of Catalytic and Antibacterial Activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014;130:54–58. doi: 10.1016/j.saa.2014.03.070. [DOI] [PubMed] [Google Scholar]
- 164.Bindhu M.R., Umadevi M. Silver and Gold Nanoparticles for Sensor and Antibacterial Applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014;128:37–45. doi: 10.1016/j.saa.2014.02.119. [DOI] [PubMed] [Google Scholar]
- 165.Mishra A., Kumari M., Pandey S., Chaudhry V., Gupta K.C., Nautiyal C.S. Biocatalytic and Antimicrobial Activities of Gold Nanoparticles Synthesized by Trichoderma sp. Bioresour. Technol. 2014;166:235–242. doi: 10.1016/j.biortech.2014.04.085. [DOI] [PubMed] [Google Scholar]
- 166.Geethalakshmi R., Sarada D.V.L. Characterization and Antimicrobial Activity of Gold and Silver Nanoparticles Synthesized Using Saponin Isolated from Trianthema decandra L. Ind. Crops Prod. 2013;51:107–115. doi: 10.1016/j.indcrop.2013.08.055. [DOI] [Google Scholar]
- 167.Velmurugan P., Anbalagan K., Manosathyadevan M., Lee K.-J., Cho M., Lee S.-M., Park J.-H., Oh S.-G., Bang K.-S., Oh B.-T. Green Synthesis of Silver and Gold Nanoparticles Using Zingiber officinale Root Extract and Antibacterial Activity of Silver Nanoparticles against Food Pathogens. Bioprocess Biosyst. Eng. 2014;37:1935–1943. doi: 10.1007/s00449-014-1169-6. [DOI] [PubMed] [Google Scholar]
- 168.Sadeghi B. Zizyphus mauritiana Extract-Mediated Green and Rapid Synthesis of Gold Nanoparticles and Its Antibacterial Activity. J. Nanostructure Chem. 2015;5:265–273. doi: 10.1007/s40097-015-0157-y. [DOI] [Google Scholar]
- 169.Vijayashree I., Niranjana P., Prabhu G., Sureshbabu V., Manjanna J. Conjugation of Au Nanoparticles with Chlorambucil for Improved Anti-Cancer Activity. J. Clust. Sci. 2017;28:133–148. doi: 10.1007/s10876-016-1053-4. [DOI] [Google Scholar]
- 170.Green R.J. Green Phyto-Synthesis Of Gold Nanoparticles Using Achyranthes aspera Linn Seed-Epicotyls Layer Extracts And Its Anticancer Activity. Asian J. Pharm. Clin. Res. 2014;7:136–139. [Google Scholar]
- 171.Sathishkumar G., Jha P.K., Vignesh V., Rajkuberan C., Jeyaraj M., Selvakumar M., Jha R., Sivaramakrishnan S. Cannonball Fruit (Couroupita guianensis, Aubl.) Extract Mediated Synthesis of Gold Nanoparticles and Evaluation of Its Antioxidant Activity. J. Mol. Liq. 2016;215:229–236. doi: 10.1016/j.molliq.2015.12.043. [DOI] [Google Scholar]
- 172.Geetha R., Ashokkumar T., Tamilselvan S. Green Synthesis of Gold Nanoparticles and Their Anti-Cancer Activity. Cancer Nanotechnol. 2013;4:91–98. doi: 10.1007/s12645-013-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu R., Pei Q., Shou T., Zhang W., Hu J., Li W. Apoptotic Effect of Green Synthesized Gold Nanoparticles from Curcuma wenyujin Extract against Human Renal Cell Carcinoma A498 Cells. Int. J. Nanomed. 2019;14:4091–4103. doi: 10.2147/IJN.S203222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ismail E.H., Saqer A.M.A., Assirey E., Naqvi A., Okasha R.M. Successful Green Synthesis of Gold Nanoparticles Using a Corchorus olitorius Extract and Their Antiproliferative Effect in Cancer Cells. Int. J. Mol. Sci. 2018;19:2612. doi: 10.3390/ijms19092612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sun B., Hu N., Han L., Pi Y., Gao Y., Chen K. Anticancer Activity of Green Synthesised Gold Nanoparticles from Marsdenia tenacissima Inhibits A549 Cell Proliferation through the Apoptotic Pathway. Artif. Cells Nanomed. Biotechnol. 2019;47:4012–4019. doi: 10.1080/21691401.2019.1575844. [DOI] [PubMed] [Google Scholar]
- 176.Balashanmugam P., Mosachristas K., Kowsalya E. In Vitro Cytotoxicity And Antioxidant Evaluation Of Biogenic Synthesized Gold Nanoparticles From Marsilea quadrifolia On Lung And Ovarian Cancer Cells. Int. J. Appl. Pharm. 2018;10:153–158. [Google Scholar]
- 177.Philip D. Rapid Green Synthesis of Spherical Gold Nanoparticles Using Mangifera indica Leaf. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010;77:807–810. doi: 10.1016/j.saa.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 178.Tahir K., Nazir S., Li B., Khan A.U., Khan Z.U.H., Gong P.Y., Khan S.U., Ahmad A. Nerium oleander Leaves Extract Mediated Synthesis of Gold Nanoparticles and Its Antioxidant Activity. Mater. Lett. 2015;156:198–201. doi: 10.1016/j.matlet.2015.05.062. [DOI] [Google Scholar]
- 179.Al-Sheddi E.S., Farshori N.N., Al-Oqail M.M., Al-Massarani S.M., Saquib Q., Wahab R., Musarrat J., Al-Khedhairy A.A., Siddiqui M.A. Anticancer Potential of Green Synthesized Silver Nanoparticles Using Extract of Nepeta deflersiana against Human Cervical Cancer Cells (HeLA) Bioinorg. Chem. Appl. 2018;2018:9390784. doi: 10.1155/2018/9390784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yas R.M., Ghafoor A., Saeed M.A. Anticancer Effect of Green Synthesized Gold Nanoparticles Using Orchid Extract and Their Characterizations on Breast Cancer AMJ-13 Cell Line. Syst. Rev. Pharm. 2021;12:500–505. [Google Scholar]
- 181.Leonard K., Ahmmad B., Okamura H., Kurawaki J. In Situ Green Synthesis of Biocompatible Ginseng Capped Gold Nanoparticles with Remarkable Stability. Colloids Surf. B Biointerfaces. 2011;82:391–396. doi: 10.1016/j.colsurfb.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 182.Rajeshkumar S., Kumar S.V., Malarkodi C., Vanaja M., Paulkumar K., Annadurai G. Optimized Synthesis of Gold Nanoparticles Using Green Chemical Process and Its Invitro Anticancer Activity Against HepG2 and A549 Cell Lines. Mech. Mater. Sci. Eng. J. 2017;9 [Google Scholar]
- 183.Wang L., Xu J., Yan Y., Liu H., Karunakaran T., Li F. Green Synthesis of Gold Nanoparticles from Scutellaria barbata and Its Anticancer Activity in Pancreatic Cancer Cell (PANC-1) Artif. Cells Nanomed. Biotechnol. 2019;47:1617–1627. doi: 10.1080/21691401.2019.1594862. [DOI] [PubMed] [Google Scholar]
- 184.Hoshyar R., Khayati G.R., Poorgholami M., Kaykhaii M. A Novel Green One-Step Synthesis of Gold Nanoparticles Using Crocin and Their Anti-Cancer Activities. J. Photochem. Photobiol. B: Biol. 2016;159:237–242. doi: 10.1016/j.jphotobiol.2016.03.056. [DOI] [PubMed] [Google Scholar]
- 185.Dhas T.S., Kumar V.G., Karthick V., Govindaraju K., Narayana T.S. Biosynthesis of Gold Nanoparticles Using Sargassum swartzii and Its Cytotoxicity Effect on HeLa Cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014;133:102–106. doi: 10.1016/j.saa.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 186.Namvar F., Mohamad R., Shameli K., Rahman H.S. Green Synthesis of Gold Nanoparticles (GNP) Using Seaweed Extract and Its Anticancer Effect. Open Conf. Proc. J. 2013;4:74. doi: 10.2174/2210289201304010074. [DOI] [Google Scholar]
- 187.Kajani A.A., Bordbar A.-K., Zarkesh Esfahani S.H., Razmjou A. Gold Nanoparticles as Potent Anticancer Agent: Green Synthesis, Characterization, and in Vitro Study. RSC Adv. 2016;6:63973–63983. doi: 10.1039/C6RA09050H. [DOI] [Google Scholar]
- 188.Dey A., Yogamoorthy S.M. Sundarapandian Green Synthesis of Gold Nanoparticles and Evaluation of Its Cytotoxic Property Against Colon Cancer Cell Line. Pharm. Chem. Sci. 2018;4:1–17. [Google Scholar]
- 189.Patra B., Gautam R., Priyadarsini E., Rajamani P., Pradhan S.N., Saravanan M., Meena R. Piper Betle: Augmented Synthesis of Gold Nanoparticles and Its in-Vitro Cytotoxicity Assessment on HeLa and HEK293 Cells. J. Clust. Sci. 2020;31:133–145. doi: 10.1007/s10876-019-01625-5. [DOI] [Google Scholar]
- 190.Jayaseelan C., Ramkumar R., Rahuman A.A., Perumal P. Green Synthesis of Gold Nanoparticles Using Seed Aqueous Extract of Abelmoschus esculentus and Its Antifungal Activity. Ind. Crops Prod. 2013;45:423–429. doi: 10.1016/j.indcrop.2012.12.019. [DOI] [Google Scholar]
- 191.Sundararajan B., Kumari B.R. Novel Synthesis of Gold Nanoparticles Using Artemisia vulgaris L. Leaf Extract and Their Efficacy of Larvicidal Activity against Dengue Fever Vector Aedes aegypti L. J. Trace Elem. Med. Biol. 2017;43:187–196. doi: 10.1016/j.jtemb.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 192.Mobaraki F., Momeni M., Taghavizadeh Yazdi M.E., Meshkat Z., Silanian Toosi M., Hosseini S.M. Plant-Derived Synthesis and Characterization of Gold Nanoparticles: Investigation of Its Antioxidant and Anticancer Activity against Human Testicular Embryonic Carcinoma Stem Cells. Process Biochem. 2021;111:167–177. doi: 10.1016/j.procbio.2021.09.010. [DOI] [Google Scholar]
- 193.Mousavi-Kouhi S.M., Beyk-Khormizi A., Mohammadzadeh V., Ashna M., Es-haghi A., Mashreghi M., Hashemzadeh V., Mozafarri H., Nadaf M., Yazdi M.E.T. Biological Synthesis and Characterization of Gold Nanoparticles Using Verbascum speciosum Schrad. and Cytotoxicity Properties toward HepG2 Cancer Cell Line. Res. Chem. Intermed. 2022;48:167–178. doi: 10.1007/s11164-021-04600-w. [DOI] [Google Scholar]
- 194.Yazdi M.T., Nazarnezhad S., Mousavi S., Amiri M.S., Darroudi M., Baino F., Kargozar S. Gum Tragacanth (GT): A Versatile Biocompatible Material beyond Borders. Molecules. 2021;26:1510. doi: 10.3390/molecules26061510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Akhter S., Ahmad M.Z., Ahmad F.J., Storm G., Kok R.J. Gold Nanoparticles in Theranostic Oncology: Current State of the Art. Expert Opin. Drug Deliv. 2021;10:1225–1243. doi: 10.1517/17425247.2012.716824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.