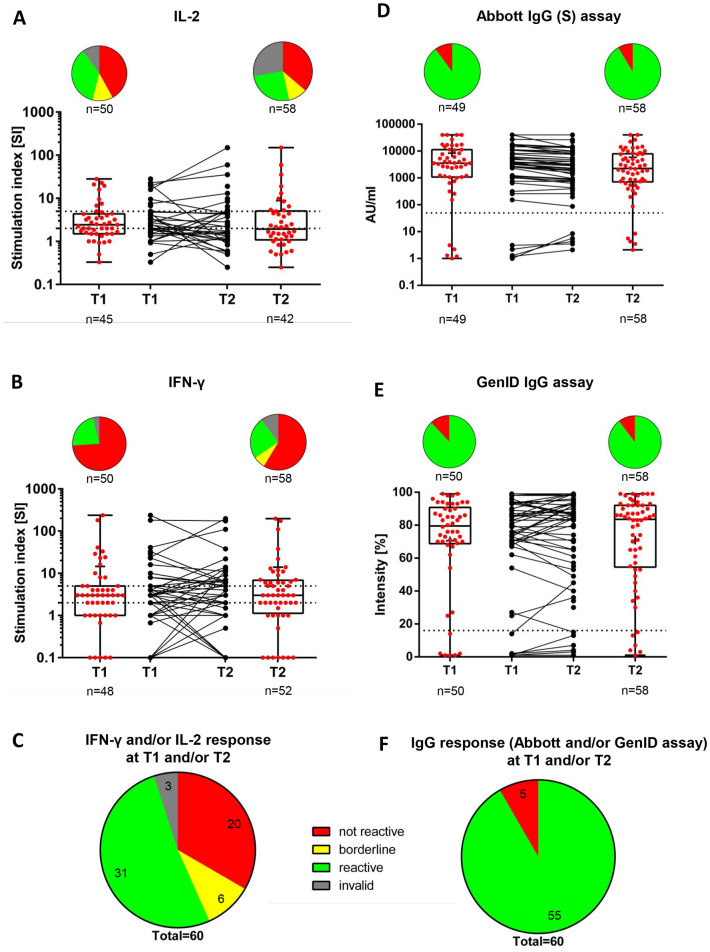
Fig. 1.
Vaccine-induced SARS-CoV-2-specific IL-2 (A) and IFN-γ (B) and IL-2 and/or IFN-γ (C)-producing T cells and SARS-CoV-2 anti-spike IgG antibodies as determined using the Abbott assay (D), GenID assay (E), and both (F) at T1 and T2.
A) T1 (n = 50): Not reactive, 21 (42.0%); borderline, 6 (12.0%); reactive, 18 (36.0%); invalid, 5 (10.0%). T2 (n = 58): Not reactive, 21 (36.2%); borderline, 6 (10.3%); reactive, 15 (25.9%); invalid, 16 (27.6%). T1 vs. T2 (p = 0.73).
B) T1 (n = 50): Not reactive, 37 (74.0%); borderline, 0 (0.0%); reactive, 11 (22.0%); invalid, 2 (4.0%). T2 (n = 58): Not reactive, 34 (58.6%); borderline, 4 (6.9%); reactive, 14 (24.1%); invalid, 6 (10.3%). T1 vs. T2 (p = 0.12).
C) T1 and/or T2 (n = 60): Not reactive, 20 (33.3%); borderline, 6 (10.0%); reactive, 31 (51.7%); invalid, 3 (5.0%).
D) T1 (n = 50): Positive, 44 (88.0%)a; negative, 6 (12.0%)b. T2 (n = 58): Positive, 52 (89.7%)a; negative, 6 (10.3%)b. T1 vs. T2 (p = 1.0)e.
E) T1 (n = 49): Positive, 44 (89.8%)c; negative, 5 (10.2%)d. T2 (n = 58): Positive, 53 (91.4%)c; negative, 5 (8.6%)d. T1 vs. T2 (p = NS)e.
F) T1 and/or T2 (n = 60): Positive, 55 (91.7%); negative, 5 (8.3%).
The dashed horizontal lines indicate the cut-off for positivity (reactive); the area between the horizontal lines indicates the borderline zone used in each GenID assay.
aPositive refers to antibody levels >16%.
bNegative refers to antibody levels ≤16%.
cPositive refers to antibody concentration > 50 AU/mL.
dNegative refers to antibody concentration ≤ 50 AU/mL.
eMcNemar's test for paired nominal data was used.
IFN-γ, interferon-γ; IL-2, interleukin-2; SARS-CoV-2, NS, not significant; severe acute respiratory syndrome-coronavirus type-2; T1, timepoint 1; T2, timepoint 2.