Abstract
Purpose
To summarize the current evidence on COVID-19 vaccine-associated ocular adverse events.
Design
Narrative literature review.
Methods
The literature search was conducted in August 2021 using 4 electronic databases: MEDLINE, EMBASE, PubMed, and the Cochrane Database of Systematic Reviews. Population-based pharmacovigilance surveillance data were retrieved from all governmental agencies participating in the World Health Organization (WHO) Programme for International Drug Monitoring with publicly available online adverse event databases in English.
Results
A small number of case reports have documented uveitis flares and acute corneal graft rejection occurring within the first 3 weeks following immunization, while isolated cases of optic neuropathies, retinal conditions, scleritis, and herpetic eye disease have also been highlighted. However, data from population-based pharmacovigilance surveillance systems suggest that the prevalence of vaccination-associated ocular adverse events are very rare.
Conclusions
Vaccination-associated ocular adverse events are rare, and there is currently no substantive evidence to counterweigh the overwhelming benefits of COVID-19 immunization in patients with pre-existing ophthalmic conditions.
INTRODUCTION
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak in December 2019 led to the devastating coronavirus disease 2019 (COVID-19) pandemic, which has become one of the largest global health threats to date.1, 2, 3 As of late August 2021, almost 220 million people had been infected worldwide, and more than 4.5 million lives claimed.2 However, the isolation of the SARS-CoV-2 virus and subsequent publication of its genome sequence in January 2020 ignited an unprecedented global response towards the accelerated development of preventative vaccine candidates, and mobilized multinational collaborative efforts between pharmaceutical corporations, academic institutions, and governmental agencies.4 , 5 The expedited process culminated in 2 recombinant mRNA vaccines (Pfizer/BioNTech BNT162b2, and Moderna mRNA-1273) being granted emergency use authorization by the United States Food and Drug Administration in December 2020.6 A number of other immunizations, including 2 adenovirus vector-based vaccines (AstraZeneca ChAdOx1 nCoV-19 and Janssen Ad26.COV2.S),6 have subsequently been approved worldwide, and more than 5.3 billion COVID-19 vaccination doses have been administered globally to date.2
Large phase 3 clinical trials have confirmed the high protective efficacy levels of several approved vaccines against serious COVID-19 infection, as well as demonstrating favorable safety profiles and low incidence rates of major adverse events.7, 8, 9, 10 Nevertheless, the inherent methodological limitations of phase 3 clinical trials–including sample size, follow-up duration, and enrolment criteria restrictions–would preclude dedicated evaluation of rare and serious adverse vaccine-associated outcomes.11 Population-based pharmacovigilance surveillance systems have therefore become necessary to enable ongoing monitoring of vaccine safety.11 , 12 A number of rare systemic adverse events following COVID-19 immunization have since garnered considerable attention, including: cerebral venous thrombosis, immune thrombocytopenia, and acute myocarditis.13, 14, 15 However, there is a significant paucity of scientific evidence evaluating the potential adverse effects of COVID-19 vaccines to the eye.16 Therefore, this narrative literature review aimed provide an overview of the current evidence on COVID-19 vaccine-associated ocular adverse events, as well as the clinical safety in patients with pre-existing ophthalmic conditions, using population-based pharmacovigilance surveillance systems and data reported in the peer-reviewed literature.
METHOD OF LITERATURE SEARCH
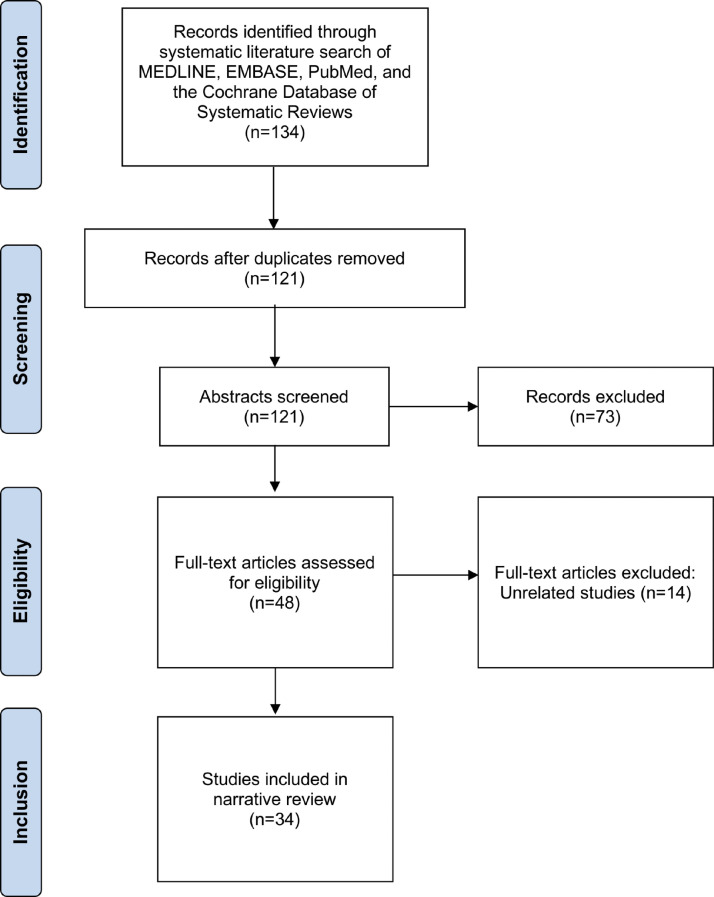
The literature search was conducted on August 31, 2021 (Figure 1 ) using 4 electronic databases: MEDLINE, EMBASE, PubMed, and the Cochrane Database of Systematic Reviews. Appropriate keywords, subject headings, free text, and thesaurus search terms were used in order to maximize sensitivity for the search strategy, and included the following terms used in various combinations: “coronavirus, COVID-19, SARS-CoV-2, 2019-nCoV, vaccine, vaccination, immunization, eye, cornea, herpes simplex, herpes zoster, uveitis, scleritis, episcleritis, retina, retinopathy, retinal degeneration, macula, maculopathy, macular degeneration, optic nerve, optic neuropathy, optic neuritis, glaucoma”. No time range limits were set for the literature search. Published English abstracts for articles written in other languages were reviewed where available. The reference lists of retrieved articles were also reviewed for further identification of potentially relevant studies. A summary of all included studies from the peer-reviewed literature is provided in Table 1 .
Figure 1.
Summary of systematic search of peer-reviewed literature.
Table 1.
Summary of Adverse Ocular Events Following COVID-19 Vaccination Reported In the Peer-Reviewed Literature
| Study | Patients | Vaccine | Presentation | Clinical Outcomes |
|---|---|---|---|---|
| Ocular Inflammatory Disease | ||||
| ElSheikh et al. 202124 | 1 | BBIBP-CorV (Sinopharm): second dose administered 5 days earlier in a single patient. | Anterior uveitis in single patient with background of antinuclear, antibody-positive, oligoarticular, juvenile idiopathic arthritis, but no previous history of uveitis. | Initial treatment with 2-hourly topical prednisolone therapy and topical cyclopentolate 2 times daily was commenced. Complete resolution and return to baseline visual acuity occurred within 6 weeks. |
| Furer et al. 202123 | 2 | BNT162b2 (Pfizer/BioNTech): vaccination dose details not documented. | Uveitis in 2 patents with background of autoimmune inflammatory rheumatic diseases. Further clinical details not documented. | Not documented. |
| Goyal et al. 202125 | 1 | ChAdOx1 nCoV-19 (AstraZeneca): second dose administered 9 days earlier in a single patient. | Multifocal choroiditis with no previous history of systemic or ocular inflammatory conditions. | Initial treatment with oral prednisone 100 mg daily was commenced, with tapering course by 10 mg/week. Clinical outcome and follow-up not documented. |
| Mudie et al. 202127 | 1 | BBIBP-CorV (Sinopharm): second dose administered 3 days earlier in a single patient. | Panuveitis in a single patient. Past ocular and systemic history of the patient was not documented. | Initial treatment with topical difluprednate therapy 4 times daily and cycloplegia for anterior and intermediate uveitis. Commenced on oral prednisone 50 mg daily and 2-hourly topical difluprednate the following day for panuveitis. Oral prednisone subsequently tapered over 3 weeks with return of visual acuity to baseline. However, at 6-week follow-up, despite maintaining baseline visual acuity, the patient developed new floaters and recurrence of mild choroidal thickening, and was subsequently re-started on high-dose oral prednisone with plan for extended taper. |
| Papasavvas et al. 202128 | 1 | BBIBP-CorV (Sinopharm): second dose administered 6 weeks earlier in a single patient. | Vogt-Koyanagi-Harada disease reactivation in a single patient, with significant anterior segment inflammation, retinal folds, and subretinal fluid. The patient had previously been well controlled on maintenance treatment with 10-weekly infliximab for the past 6 years. | Initial treatment with a 5-day course of oral prednisone (1 mg/kg) and loading dose scheme of infliximab administered, with short-term clinical improvements recorded. |
| Pichi et al. 202129 | 3 | BBIBP-CorV (Sinopharm): unspecified dose administered 1 week earlier in 1 patient, and up to 15 days earlier in the other 2 patients with no further clinical details recorded. | Anterior scleritis in 2 patients, and episcleritis in 1 patient. One of the patients with anterior scleritis had a history of rheumatoid arthritis on sulfasalazine therapy. Clinical details not documented for the other 2 patients. | Initial treatment with 1-week tapering course of topical corticosteroid therapy of unspecified dose commenced in 1 patient, with clinical resolution occurring in 1 week, without the requirement for systemic corticosteroid treatment. No clinical follow-up details documented for the other 2 patients. |
| Rabinovitch et al. 202126 | 21 | BNT162b2 (Pfizer/BioNTech): first dose administered 1 to 14 days earlier in 8 patients, and second dose administered 1 to 30 days earlier in 13 patients. | Anterior uveitis in 18 patients, multiple evanescent white dot syndrome in 2 patients, anterior and intermediate uveitis with cystoid macular edema in 1 patient. Seven patients had previous history of anterior uveitis, and 1 patient had previous history of herpes zoster ophthalmicus keratouveitis. Three patients had ankylosing spondylitis, 1 patient had Crohn's disease, and 1 patient had psoriasis. Twelve patients had no previous history of systemic or ocular inflammatory conditions. | Initial treatment with topical prednisolone (3-hourly to hourly) or dexamethasone (4 times daily to 2-hourly), and topical cyclopentolate (daily to 3 times daily) or tropicamide (daily to 3 times daily) was commenced in the 18 patients with anterior uveitis. Initial treatment with hourly topical prednisolone therapy and topical cyclopentolate 3 times daily was commenced in the patient with anterior and intermediate uveitis with cystoid macular edema, intravitreal dexamethasone injection (Ozurdex) was subsequently administered. The 2 patients with multiple evanescent white dot syndrome were managed conservatively. Complete resolution or significant improvement with return to baseline visual acuity occurred in all patients, although the time frame was not documented. |
| Renisi et al. 202140 | 1 | BNT162b2 (Pfizer/BioNTech): second dose administered 14 days earlier in a single patient. | Anterior uveitis in a single patient with no previous history of systemic or ocular inflammatory conditions. | Initial treatment with topical dexamethasone therapy 3 times daily and topical atropine twice daily was commenced, although topical dexamethasone was uptitrated to 6 times daily the following week before subsequent tapering. Complete resolution and return to baseline visual acuity occurred within 6 weeks. |
| Herpetic Eye Disease | ||||
| Furer et al. 202131 | 1 | BNT162b2 (Pfizer/BioNTech): first dose administered 4 days earlier in a single patient. | First episode of herpes zoster ophthalmicus with conjunctivitis in a patient with background of rheumatoid arthritis on tofacitinib therapy. | Initial treatment with 14-day course of oral acyclovir treatment and topical lubricant eye drops, and temporary discontinuation of tofacitinib therapy. Resolution occurred within 6 weeks. |
| Richardson-May et al. 202134 | 1 | ChAdOx1 nCoV-19 (AstraZeneca): first dose administered 1 day earlier in a single patient. | Recurrence of herpes simplex stromal keratitis in a patient with background of herpes zoster keratitis 40 years earlier. | Initial treatment with topical ganciclovir for herpes simplex epithelial keratitis. However, clinical deterioration noted at 7 days with geographical ulceration, corneal haze, and mild anterior chamber inflammation. Corneal scrape and viral PCR subsequently confirmed a diagnosis of herpes simplex stromal keratitis. Clinical improvements were observed with systemic antiviral and topical corticosteroid and antibiotic therapy, although residual corneal scarring was observed at 6-week follow-up. |
| Thimmanagari et al. 202132 | 2 | mRNA-1273 (Moderna): first dose administered 1 week earlier in first patient. Ad26.COV2.S (Janssen): administered 1 week earlier in second patient. |
First episode herpes zoster ophthalmicus with conjunctivitis in first patient, and no ophthalmic involvement in second patient. Both patients had no previous history of systemic inflammatory conditions or immunosuppressant therapy. | Systemic antiviral therapy commenced in both patients, although medication dosage not documented. Resolution reported in both patients, although timeframe of clinical follow-up not documented. |
| Anterior Segment Conditions | ||||
| Crnej et al. 202135 | 1 | BNT162b2 (Pfizer/BioNTech): first dose administered 7 days earlier in a single patient. | Acute corneal graft rejection in a single patients with Descemet membrane endothelial keratoplasty for post-cataract surgery endothelial decompensation. | Initial treatment with 2-hourly topical dexamethasone therapy and oral valacyclovir 1 g 3 times daily was commenced in the patient. Significant improvements and return to baseline visual acuity occurred within 7 days. |
| Phylactou et al. 202136 | 2 | BNT162b2 (Pfizer/BioNTech): first dose administered 7 days earlier in the first patient, and second dose administered 3 weeks earlier in second patient. | Acute corneal graft rejection in 2 patients with Descemet membrane endothelial keratoplasty or Fuchs’ endothelial corneal dystrophy. | Initial treatment with hourly topical dexamethasone therapy was commenced in both patients. Significant improvements and return to baseline visual acuity occurred in both cases within 7 days. |
| Rallis et al. 202137 | 1 | BNT162b2 (Pfizer/BioNTech): first dose administered 4 days earlier in a single patient. | Acute corneal graft rejection in a single patient with penetrating keratoplasty for Fuchs’ endothelial corneal dystrophy. | Initial treatment with hourly topical dexamethasone therapy and 1-week course of oral acyclovir 400 mg 5 times daily was commenced in the patient. Significant improvements and return to baseline visual acuity occurred within 3 weeks. |
| Ravichandran et al. 202138 | 1 | ChAdOx1 nCoV-19 (AstraZeneca): first dose administered 3 weeks earlier in a single patient. | Acute corneal graft rejection in a single patient with penetrating keratoplasty for childhood corneal scar. | Not documented. |
| Wasser et al. 202139 | 2 | BNT162b2 (Pfizer/BioNTech): first dose administered 13 and 14 days earlier, respectively, in the 2 patients. | Acute corneal graft rejection in 2 patients with penetrating keratoplasty for keratoconus. | Initial treatment with topical dexamethasone therapy and oral prednisone 60 mg daily was commenced in both patients. Significant improvements and return to baseline visual acuity occurred within 7 days in the first patient, and 2 weeks in the second patient. |
| Retinal Conditions | ||||
| Book et al. 202147 | 1 | ChAdOx1 nCoV-19 (AstraZeneca): first dose administered 3 days earlier in a single patient. | Acute macular neuroretinopathy in a single patient with no significant past ocular history. | Not documented. |
| Bøhler et al. 202148 | 1 | ChAdOx1 nCoV-19 (AstraZeneca): first dose administered 2 days earlier in a single patient. | Acute macular neuroretinopathy in a single patient on combined desogestrel and ethinylestradiol contraceptive therapy, with no significant past ocular history. | Not documented. |
| Maleki et al. 202150 | 1 | mRNA-1273 (Moderna): second dose administered 10 days earlier in a single patient. | Acute zonal occult outer retinopathy in a single patient with background of pre-eclampsia and 1 previous unexplained miscarriage, but no significant past ocular history. | Treated with intravitreal dexamethasone implant, and combination azathioprine and cyclosporine therapy was considered, although further clinical follow-up details were not documented. |
| Mambretti et al. 202149 | 2 | ChAdOx1 nCoV-19 (AstraZeneca): first dose administered 2 days earlier in 2 patients. | Acute macular neuroretinopathy in 2 patients, both on long-term oral contraceptive therapy, with no significant past ocular history. | Not documented. |
| Pichi et al. 202129 | 4 | BBIBP-CorV (Sinopharm): unspecified dose administered 5 days earlier in 1 patient, and up to 15 days earlier in the other 3 patients with no further clinical details recorded. | Acute macular neuroretinopathy in 2 patients, paracentral acute middle maculopathy in 1 patient, subretinal fluid thought to represent forme fruste central serous chorioretinopathy in 1 patient. One of the patients with acute macular neuroretinopathy had previous ocular history of central serous chorioretinopathy, while the other patient with acute macular neuroretinopathy had no significant past ocular or systemic history. Clinical details were not documented for the other 2 patients. | One of the patients with acute macular neuroretinopathy was managed conservatively, and the optical coherence tomographic lesions were reported to resolve spontaneously at 2 months. No clinical follow-up details were documented for the remaining 3 patients. |
| Optic Neuropathy | ||||
| Helmchen et al. 202151 | 1 | ChAdOx1 nCoV-19 (AstraZeneca) vaccine: first dose administered 2 weeks earlier in a single patient. | Seronegative neuromyelitis optica spectrum disorder with bilateral optic neuritis with chiasmal involvement and longitudinal extensive transverse myelitis in a single patient with background of relapsing-remittent multiple sclerosis (on natalizumab therapy. | Initial treatment with intravenous methylprednisolone 2 g daily for 2 days, followed by plasmapheresis and immunoadsorption with mild improvement in visual acuities with recognition of motion. However, there was persistence of paraplegia, loss of sensory function below T5, and incontinence at 2 months |
| Leber et al. 202152 | 1 | CoronaVac (Sinovac) vaccine: second dose administered 12 hours earlier in single patients. | Myelin oligodendrocyte glycoprotein antibody-associated bilateral anterior optic neuritis and concurrent subacute thyroiditis in single patient. Past ocular and systemic history of the patient was not documented. | Initial treatment with intravenous methylprednisolone 1 g daily for 5 days, followed by tapering course of oral corticosteroids. Improvements in optic disc swelling, recovery of visual acuity and fields to baseline levels, and normalization of thyroid function occurred within 1 week. |
| Maleki et al. 202150 | 1 | BNT162b2 (Pfizer/BioNTech) vaccine: second dose administered 2 days earlier in a single patient | Temporal artery biopsy-positive bilateral arteritic anterior ischemic optic neuropathy in a single patient with no previous history of systemic or ocular inflammatory conditions. | Initial treatment with oral prednisone 60 mg daily and subcutaneous tocilizumab 162 mg weekly was commenced. Further clinical follow-up details were not documented. |
| Other Ophthalmic Conditions | ||||
| Bayas et al. 202160 | 1 | ChAdOx1 nCoV-19 (AstraZeneca) vaccine: first dose administered 10 days earlier in a single patient. | Vaccine-induced immune thrombotic thrombocytopenia with superior ophthalmic vein thrombosis in a single patient with no significant ocular or systemic history. | Initial treatment with intravenous dexamethasone and heparin. Developed left parietal lobe ischemic stroke 8 days following admission, followed by right-sided seizures controlled by levetiracetam and lacosamide. Heparin subsequently switched to phenprocoumon, and patient discharged 25 days following admission. |
| Burrows et al. 202171 | 1 | BNT162b2 (Pfizer/BioNTech) vaccine: first dose administered 5 days prior to right-sided Bell's palsy, and second dose administered 2 days prior to left-sided Bell's palsy. | Bilateral sequential Bell's palsy in a single patient with a background of hypertension, hypercholesterolemia, and type 2 diabetes. | Initial treatment with oral prednisone 60 mg daily subsequently weaned over a 4-week period, with resolution of right-sided Bell's palsy. Re-presented 2 days following second vaccine dose with left-sided Bell's palsy, and treated with 7-day course of prednisone 60 mg daily and weaned over a 4-week period, with resolution of left-sided Bell's palsy. |
| Colella et al. 202172 | 1 | BNT162b2 (Pfizer/BioNTech) vaccine: first dose administered 5 days earlier. | Unilateral Bell's palsy in a single patient with no significant ocular or systemic history. | Initial treatment with oral prednisone 50 mg daily and artificial tears. Further details surrounding treatment taper not provided. At 1 month follow-up, partial improvement in facial mobility was observed, although pain sensation persisted. |
| El-Shitany et al. 202177 | 3 | BNT162b2 (Pfizer/BioNTech) vaccine: adverse events occurred in 3 cases following the first dose, further details not provided. | Bell's palsy in 3 patients. Further clinical details not provided. | Not documented. |
| Iftikhar et al. 202173 | 1 | mRNA-1273 (Moderna) vaccine: second dose administered 2 days earlier. | Unilateral Bell's palsy in a single patient with no significant ocular or systemic history. | Initial treatment with 7-day course of oral prednisone 60 mg daily and artificial tears. Improvements observed at 2 week follow-up, no further clinical follow-up details provided. |
| Mason et al. 202174 | 1 | mRNA-1273 (Moderna) vaccine: dose administered 4 weeks earlier, although unclear whether the first or second dose. | Bilateral sequential Bell's palsy in a single patient with a background of migraine headaches. | Initial treatment plan for intravenous 500 mg twice daily for 3 days followed by oral prednisone taper, and oral acyclovir 400 mg 4 times daily for 10 days, after negative MRI brain and CT angiography. Four days later developed contralateral Bell's palsy, with negative lumbar puncture and MRI brain with contrast. Re-commenced on 500 mg twice daily for 3 days with significant improvement and discharged on oral prednisone taper. |
| Martin-Villares et al. 202175 | 1 | mRNA-1273 (Moderna) vaccine: first dose administered 2 days earlier. | Unilateral Bells’ palsy in a single patient with 1 previous episode of Bell's palsy during pregnancy 9 years ago. | Treatment details not provided. Full resolution of unilateral Bell's palsy occurred at 3-week follow-up. |
| Panovska-Stavridis et al. 202159 | 1 | ChAdOx1 nCoV-19 (AstraZeneca) vaccine: first dose administered 10 days earlier in a single patient. | Vaccine-induced immune thrombotic thrombocytopenia with superior ophthalmic vein thrombosis in a single patient with no significant ocular or systemic history. | Initial treatment with a 2-day course of intravenous immunoglobulin followed by a tapering course of oral corticosteroid therapy, and commenced on rivaroxaban anticoagulation. Symptomatic resolution occurred within 5 days, platelet levels normalized in 1 week, and D-dimer levels normalized in 2 weeks. |
| Repajic et al. 202176 | 1 | BNT162b2 (Pfizer/BioNTech) vaccine: second dose administered 2 days earlier. | Unilateral Bells’ palsy in a single patient with a background of hypertension and recurrent Bell's palsy. | Treatment details not provided. Clinical improvement in unilateral Bell's palsy observed at 2-week follow-up. |
| Reyes-Capo et al. 202165 | 1 | BNT162b2 (Pfizer/BioNTech) vaccine: dose administered 2 days earlier, although unclear whether the first or second dose. | Isolated abducent nerve palsy in a single patient with no significant ocular or systemic history. | Not documented. |
| Shemer et al. 202178 | 21 | BNT162b2 (Pfizer/BioNTech) vaccine: further details regarding the timing of vaccination was not provided for the 21 patients. | Unilateral Bell's palsy in 21 patients. Four patients had a background of hypertension, 3 patients had dyslipidemia, 2 patients had diabetes, 1 patient had small-fiber neuropathy, 1 patient had a cardiac pacemaker, 1 patient had obstructive sleep apnea, 1 patient had Meniere's disease, 1 patient had asthma, 1 patient had hypothyroidism, 1 patient had thalassemia, 1 patient had prostate cancer, 1 patient had benign prostatic hypertrophy | Treatment details not provided. Partial recovery at last follow-up was documented in 9 patients. No clinical follow-up details were provided from the remaining 12 patients. |
| Wan et al. 202179 | 44 | CoronaVac (Sinovac) vaccine: first dose administered 1-42 days earlier in 19 patients, and second dose administered 1-17 days earlier in 9 patients. BNT162b2 (Pfizer/BioNTech): first dose administered 2-20 days earlier in 8 patients, and second dose administered 1-18 days earlier in 8 patients. |
Unilateral Bell's palsy in 44 patients. Previous ocular and systemic history not provided. | Treatment details not provided. Significant or full recovery at last follow-up was documented in 20 of 21 patients with details provided. No clinical follow-up details were provided from the remaining 23 patients. |
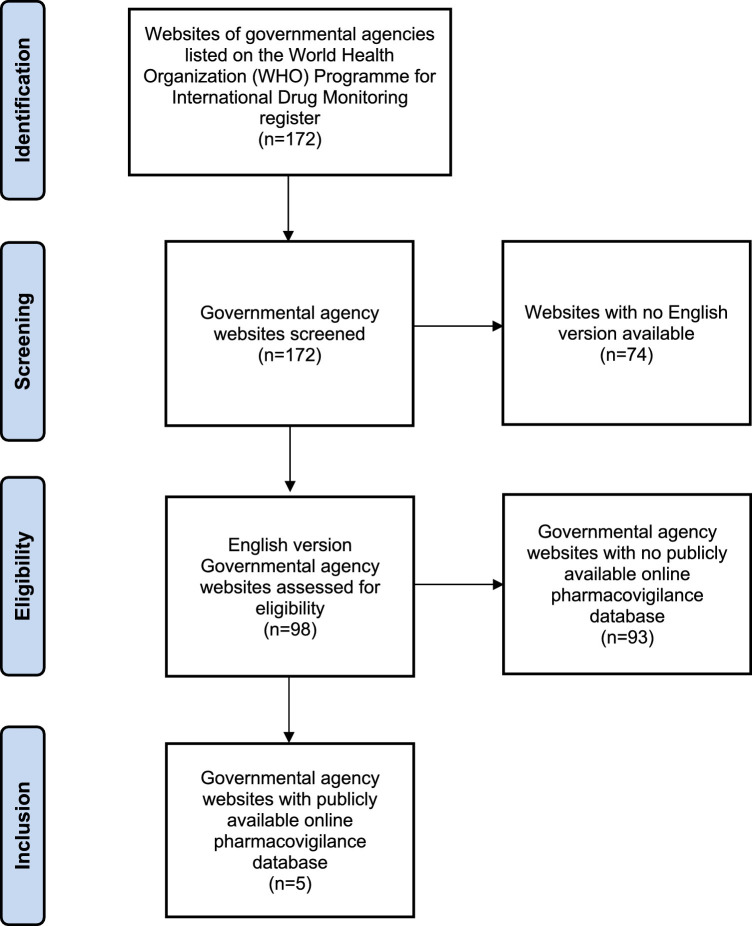
Population-based pharmacovigilance surveillance data were retrieved through searching the websites of all governmental agencies participating in the World Health Organization Programme for International Drug Monitoring with publicly available online adverse event databases in English (Figure 2 ).17 Vaccine-associated adverse event data were extracted from the government agency websites on August 31, 2021, including the Australia Therapeutic Goods Administration Database of Adverse Event Notifications,18 the Canada Vigilance Adverse Reaction Database,19 the European Union Medicines Agency EudraVigilance System,20 the United Kingdom Medicines and Healthcare Products Regulatory Agency,21 and the United States Centers for Disease Control and Prevention Vaccine Adverse Event Reporting System.22 The numbers of ocular adverse events occurring following administration of each type of vaccine were recorded. Data on vaccination dose administration were retrieved from the University of Oxford Our World in Data website on August 31, 2021, which is compiled from official reports released by national public health agencies.2 Prevalence rates of ocular adverse events were calculated by dividing the number of events by the number of vaccine doses administered. A summary of population-based pharmacovigilance surveillance data is provided in Table 2 . It is acknowledged that data generated from population-based pharmacovigilance surveillance systems can be subjected to bias secondary to the unverified nature of suspected adverse event reporting conducted by individual clinicians and patients; therefore, causal relationships and direct association cannot be inferred.
Figure 2.
Summary of systematic search of publicly available online pharmacovigilance surveillance system databases.
Table 2.
Summary of Adverse Ocular Events Following COVID–19 Vaccination Reported In Population-Based Pharmacovigilance Surveillance Systems18-22
| Adverse Ocular Event | Vaccine | European Union |
United States |
United Kingdom1 |
Canada |
Australia1 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Prevalence (Cases Per Million Doses) | Cases | Prevalence (Cases Per Million Doses) | Cases | Prevalence (Cases Per Million Doses) | Cases | Prevalence (Cases Per Million Doses) | Cases | Prevalence (Cases Per Million Doses) | ||
| Ocular Inflammatory Disease | |||||||||||
| Uveitis | Pfizer/BioNTech | 121 | 0.3 | 39 | 0.2 | 37 | – | 1 | 0.02 | 5 | – |
| Moderna | 43 | 0.8 | 40 | 0.3 | 3 | – | 0 | 0 | – | – | |
| AstraZeneca | 53 | 0.8 | – | – | 42 | – | 0 | 0 | 7 | – | |
| Janssen | 3 | 0.2 | 3 | 0.2 | – | – | – | – | – | – | |
| Unspecified | – | – | – | – | 0 | – | – | – | – | – | |
| Total | 220 | 0.4 | 82 | 0.2 | 82 | 0.9 | 1 | 0.02 | 12 | 0.6 | |
| Episcleritis | Pfizer/BioNTech | 25 | 0.07 | 19 | 0.09 | 7 | – | 0 | 0 | 0 | – |
| Moderna | 4 | 0.08 | 8 | 0.06 | 4 | – | 0 | 0 | – | – | |
| AstraZeneca | 6 | 0.09 | – | – | 18 | – | 0 | 0 | 2 | – | |
| Janssen | 1 | 0.07 | 0 | 0.00 | – | – | – | – | – | – | |
| Unspecified | – | – | – | – | 0 | – | – | – | – | – | |
| Total | 36 | 0.07 | 27 | 0.07 | 29 | 0.3 | 0 | 0 | 2 | 0.1 | |
| Scleritis | Pfizer/BioNTech | 25 | 0.07 | 11 | 0.05 | 3 | – | 0 | 0 | 1 | – |
| Moderna | 11 | 0.2 | 8 | 0.06 | 0 | – | 0 | 0 | – | – | |
| AstraZeneca | 16 | 0.2 | – | – | 13 | – | 0 | 0 | 0 | – | |
| Janssen | 1 | 0.07 | 1 | 0.07 | – | – | – | – | – | – | |
| Unspecified | – | – | – | – | 0 | – | – | – | – | – | |
| Total | 53 | 0.1 | 20 | 0.05 | 16 | 0.2 | 0 | 0 | 1 | 0.05 | |
| Herpetic Eye Disease | |||||||||||
| Ophthalmic herpes zoster | Pfizer/BioNTech | 55 | 0.1 | 0 | 0 | 3 | – | 0 | 0 | 2 | – |
| Moderna | 23 | 0.4 | 0 | 0 | 0 | – | 0 | 0 | – | – | |
| AstraZeneca | 37 | 0.5 | – | – | 10 | – | 0 | 0 | 4 | – | |
| Janssen | 2 | 0.1 | 0 | 0 | – | – | – | – | – | – | |
| Unspecified | – | – | – | – | 0 | – | – | – | – | – | |
| Total | 117 | 0.2 | 0 | 0 | 13 | 0.1 | 0 | 0 | 6 | 0.3 | |
| Ophthalmic herpes simplex | Pfizer/BioNTech | 0 | 0 | 0 | 0 | 0 | – | 0 | 0 | 0 | – |
| Moderna | 0 | 0 | 0 | 0 | 1 | – | 0 | 0 | – | – | |
| AstraZeneca | 0 | 0 | – | – | 1 | – | 0 | 0 | 1 | – | |
| Janssen | 0 | 0 | 0 | 0 | – | – | – | – | – | – | |
| Unspecified | – | – | – | – | 0 | – | – | – | – | – | |
| Total | 0 | 0 | 0 | 0 | 2 | 0.02 | 0 | 0 | 1 | 0.05 | |
| Anterior Segment Conditions | |||||||||||
| Corneal graft rejection | Pfizer/BioNTech | 10 | 0.03 | 1 | 0.005 | 5 | – | 0 | 0 | 0 | – |
| Moderna | 2 | 0.04 | 0 | 0 | 0 | – | 0 | 0 | – | – | |
| AstraZeneca | 3 | 0.04 | – | – | 3 | – | 0 | 0 | 0 | – | |
| Janssen | 0 | 0 | 0 | 0 | – | – | – | – | – | – | |
| Unspecified | – | – | – | – | 1 | – | – | – | – | – | |
| Total | 15 | 0.03 | 1 | 0.003 | 9 | 0.1 | 0 | 0 | 0 | 0 | |
| Retinal Conditions | |||||||||||
| Macular edema | Pfizer/BioNTech | 24 | 0.06 | 3 | 0.01 | 3 | – | 0 | 0 | 0 | – |
| Moderna | 10 | 0.2 | 6 | 0.04 | 1 | – | 1 | 0.06 | – | – | |
| AstraZeneca | 20 | 0.3 | – | – | 7 | – | 0 | 0 | 0 | – | |
| Janssen | 3 | 0.2 | 1 | 0.07 | – | – | – | – | – | – | |
| Unspecified | – | – | – | – | 0 | – | – | – | – | – | |
| Total | 57 | 0.1 | 10 | 0.03 | 11 | 0.1 | 1 | 0.02 | 0 | 0 | |
| Macular degeneration | Pfizer/BioNTech | 17 | 0.05 | 11 | 0.05 | 3 | – | 0 | 0 | 0 | – |
| Moderna | 5 | 0.1 | 5 | 0.03 | 0 | – | 0 | 0 | – | – | |
| AstraZeneca | 10 | 0.1 | 0 | – | 9 | – | 0 | 0 | 1 | – | |
| Janssen | 1 | 0.07 | 1 | 0.07 | – | – | – | – | – | ||
| Unspecified | – | – | – | – | 1 | – | – | – | – | – | |
| Total | 33 | 0.06 | 17 | 0.05 | 13 | 0.1 | 0 | 0 | 1 | 0.05 | |
| Maculopathy | Pfizer/BioNTech | 9 | 0.02 | 3 | 0.01 | 1 | – | 0 | 0 | 0 | – |
| Moderna | 3 | 0.06 | 0 | 0 | 0 | – | 0 | 0 | – | – | |
| AstraZeneca | 7 | 0.1 | – | – | 2 | – | 0 | 0 | 0 | – | |
| Janssen | 0 | 0 | 0 | 0 | – | – | – | – | – | – | |
| Unspecified | – | – | – | – | 0 | – | – | – | – | – | |
| Total | 19 | 0.04 | 3 | 0.008 | 3 | 0.03 | 0 | 0 | 0 | 0 | |
| Chorioretinopathy | Pfizer/BioNTech | 11 | 0.03 | 3 | 0.01 | 2 | – | 0 | 0 | 1 | – |
| Moderna | 5 | 0.1 | 2 | 0.01 | 1 | – | 0 | 0 | – | – | |
| AstraZeneca | 8 | 0.1 | – | – | 2 | – | 0 | 0 | 1 | – | |
| Janssen | 0 | 0 | 1 | 0.07 | – | – | – | – | – | ||
| Unspecified | – | – | – | – | 0 | – | – | – | – | – | |
| Total | 24 | 0.05 | 6 | 0.02 | 5 | 0.05 | 0 | 0 | 2 | 0.1 | |
| Acute zonal occult outer retinopathy | Pfizer/BioNTech | 0 | 0 | 0 | 0 | 0 | – | 0 | 0 | 0 | – |
| Moderna | 0 | 0 | 0 | 0 | 1 | – | 0 | 0 | – | – | |
| AstraZeneca | 0 | 0 | – | – | 1 | – | 0 | 0 | 0 | – | |
| Janssen | 0 | 0 | 0 | 0 | – | – | – | – | – | ||
| Unspecified | – | – | – | – | 0 | – | – | – | – | – | |
| Total | 0 | 0 | 0 | 0 | 2 | 0.02 | 0 | 0 | 0 | 0 | |
| Diabetic retinopathy | Pfizer/BioNTech | 1 | 0.003 | 0 | 0 | 0 | – | 0 | 0 | 0 | – |
| Moderna | 1 | 0.02 | 1 | 0.007 | 0 | – | 0 | 0 | – | – | |
| AstraZeneca | 1 | 0.01 | – | – | 2 | – | 0 | 0 | 0 | – | |
| Janssen | 0 | 0 | 0 | 0 | – | – | – | – | – | – | |
| Unspecified | – | – | – | – | 0 | – | – | – | – | – | |
| Total | 3 | 0.006 | 1 | 0.003 | 2 | 0.02 | 0 | 0 | 0 | 0 | |
| Optic Neuropathy | |||||||||||
| Optic neuritis | Pfizer/BioNTech | 136 | 0.4 | 44 | 0.2 | 24 | – | 0 | 0 | 4 | – |
| Moderna | 47 | 0.9 | 31 | 0.2 | 3 | – | 0 | 0 | – | – | |
| AstraZeneca | 96 | 1 | – | – | 51 | – | 0 | 0 | 7 | – | |
| Janssen | 7 | 0.5 | 7 | 0.5 | – | – | – | – | – | ||
| Unspecified | – | – | – | – | 0 | – | – | – | – | – | |
| Total | 286 | 0.6 | 82 | 0.2 | 78 | 0.9 | 0 | 0 | 11 | 0.6 | |
| Ischemic optic neuropathy | Pfizer/BioNTech | 51 | 0.1 | 10 | 0.05 | 2 | – | 0 | 0 | 0 | – |
| Moderna | 30 | 0.6 | 15 | 0.1 | 0 | – | 0 | 0 | – | – | |
| AstraZeneca | 25 | 0.4 | – | – | 8 | – | 0 | 0 | 2 | – | |
| Janssen | 3 | 0.2 | 7 | 0.5 | – | – | – | – | – | – | |
| Unspecified | – | – | – | – | 0 | – | – | – | – | – | |
| Total | 109 | 0.2 | 32 | 0.09 | 10 | 0.1 | 0 | 0 | 2 | 0.1 | |
| Glaucoma | Pfizer/BioNTech2 | 34 | 0.09 | 12 | 0.06 | 5 | – | 0 | 0 | 0 | – |
| Moderna | 10 | 0.2 | 9 | 0.06 | 0 | – | 0 | 0 | – | – | |
| AstraZeneca | 21 | 0.3 | – | – | 12 | – | 0 | 0 | 0 | – | |
| Janssen | 1 | 0.07 | 0 | 0 | – | – | – | – | – | – | |
| Unspecified | – | – | – | 0 | 0 | – | – | – | – | – | |
| Total | 66 | 0.1 | 21 | 0.06 | 17 | 0.2 | 0 | 0 | 0 | 0 | |
Data for total doses administered by vaccine type were unavailable from the United Kingdom Australia.
COVID-19 VACCINATION AND OCULAR INFLAMMATORY DISEASE
COVID-19 vaccine-associated uveitis has been reported in 27 cases in the peer-reviewed literature to date (Table 1), with onset ranging from 1 to 30 days following vaccine administration.23, 24, 25, 26, 27 Twenty-five of the 27 cases received the recombinant mRNA BNT162b2 (Pfizer/BioNTech) vaccine, while the remaining 2 cases received the adenovirus vector ChAdOx1 nCoV-19 (AstraZeneca) and inactivated virus BBIBP-CorV (Sinopharm) vaccines, respectively. Seventeen cases developed uveitis following the second dose, 8 cases following the first dose, while dose administration details were unavailable for the remaining 2 cases. Eight patients had a history of previous uveitis, 9 patients had a background of autoimmune rheumatologic disease, while 12 patients had no previous history of ocular or systemic inflammatory conditions. Human leukocyte antigen B27 testing was negative in 1 case, and the results were unavailable in the remaining 26 cases. Of the 25 cases with available clinical data, 20 patients had isolated anterior uveitis, 2 patients had multiple evanescent white dot syndrome, 1 patient had anterior and intermediate uveitis with cystoid macular edema, 1 patient had multifocal choroiditis, and 1 patient had panuveitis. Complete resolution of the uveitis flare and/or recovery to baseline visual acuity was achieved in all 22 patients treated with topical, intravitreal, and/or systemic corticosteroid therapy, while the 2 patients with multiple evanescent white dot syndrome were conservatively managed and demonstrated significant improvement with recovery to baseline visual acuity at final follow-up. Clinical follow-up details were not documented for the 3 remaining uveitis cases.
A single case of Vogt-Koyanagi-Harada disease reactivation was reported to occur in a patient 6 weeks after receiving the second dose of the BNT162b2 (Pfizer/BioNTech) vaccine, with significant anterior segment inflammation, retinal folds, and subretinal fluid.28 The patient had previously been well controlled on maintenance treatment with infliximab administration every 10 weeks for the past 6 years. Clinical improvements in the reactivation occurred following a 5-day course of high-dose corticosteroid therapy and infliximab administration.
A small case series study reported 2 cases of post-vaccination acute scleritis and 1 case of episcleritis within 15 days of inactivated virus BBIBP-CorV (Sinopharm) vaccine administration.29 Clinical follow-up details were provided for 1 of the post-vaccination scleritis cases, and resolution was reported to occur following a 1-week tapering course of topical corticosteroid drops, with no requirement for systemic corticosteroid therapy.
Although the coincidental occurrence of uveitis or scleritis following immunization could not be completely excluded in the reported cases, the possibility for the COVID-19 vaccine to trigger or exacerbate ocular inflammatory disease is not inconceivable, given the close temporal association and lack of other explanatory factors. Moreover, vaccine-associated uveitis has previously been documented to occur following administration of other types of immunizations, with a recent review identifying 289 cases of vaccine-associated uveitis being reported in the literature between 1984 to 2014.30 A wide range of immunizations have been reported to trigger uveitis flares, including hepatitis A and B, human papillomavirus, influenza, Bacillus Calmette-Guérin, measles-mumps-rubella (MMR), and varicella zoster.30 However, the pathophysiology underlying vaccine-associated ocular inflammatory disease remains to be fully understood, and a myriad of potential mechanisms have been hypothesized, including molecular mimicry between peptide fragments of the vaccine and host antigens, delayed-type hypersensitivity and immune complex deposition, as well as immune responses generated against adjuvants within the vaccine.26 , 30
Population-based adverse event reporting data show that the prevalence rates of COVID-19 vaccine-associated uveitis were ≤0.9 cases per million doses, while scleritis occurred in ≤0.2 cases per million doses, and episcleritis occurred in ≤0.3 cases per million doses (Table 2).18, 19, 20, 21, 22 In addition, a multicenter observational study comparing recombinant mRNA BNT162b2 (Pfizer/BioNTech) vaccine administration in 686 patients with autoimmune inflammatory rheumatic diseases and 121 healthy controls showed that uveitis occurred in 2 (0.3%) patients with systemic autoimmune conditions and 0 (0.0%) healthy controls.23
On balance of the currently available evidence, patients with pre-existing ocular inflammatory conditions should not be discouraged from receiving COVID-19 vaccination, particularly given that the high protective efficacy against severe infection would likely outweigh the relatively low risk of triggering a uveitis flare. Moreover, complete resolution or significant improvement were also observed in all case reports with timely commencement of standard treatment protocols.24, 25, 26 , 29 There is currently insufficient evidence to support prophylactic treatment in patients with pre-existing ocular inflammatory conditions following immunization. However, patients with a background of systemic autoimmune disease or previous uveitis could be counselled regarding the potential symptoms associated with vaccine-associated uveitis, particularly given that early recognition and treatment can yield favorable outcomes.
COVID-19 VACCINATION AND HERPETIC EYE DISEASE
Three cases of first-episode herpes zoster ophthalmicus, occurring within 4 days to 1 week following COVID-19 vaccine administration, have been reported in the peer-reviewed literature (Table 1).31 , 32 The cases were reported after receiving the first dose of the recombinant mRNA-1273 (Moderna) and BNT162b2 (Pfizer/BioNTech), and the adenovirus vector Ad26.COV2.S (Janssen) vaccines, respectively. Ophthalmic involvement was limited to conjunctivitis in 2 cases, while no ophthalmic involvement was reported in the third case, and resolution was achieved in all cases with routine systemic antiviral treatment. Although causality could not be inferred on the basis of isolated case reports, the potential for vaccine-induced immunomodulation is not inconceivable, with previous cases of herpes zoster reactivation being reported following the administration of the trivalent influenza, hepatitis A, and rabies vaccinations.33
An isolated case of herpes simplex stromal keratitis recurrence was reported to occur in a patient 1 day after receiving the first dose of the adenovirus vector ChAdOx1 nCoV-19 (AstraZeneca) vaccine.34 The patient had a history of herpes simplex keratitis 40 years previously. Clinical improvements were observed with systemic antiviral and topical corticosteroid therapy, although some residual corneal scarring developed.
Data from adverse event reporting systems show that the population prevalence rates of post-vaccination ophthalmic herpes zoster were ≤0.5 cases per million doses, and the rates of ophthalmic herpes simplex were ≤0.05 cases per million doses (Table 2).18, 19, 20, 21, 22 Overall, the very low rates of post-vaccination ophthalmic herpes zoster and simplex would suggest that there is no substantive evidence to support the requirement for prophylactic antiviral treatment for patients with previous herpetic eye disease undergoing COVID-19 immunization.
COVID-19 VACCINATION AND ANTERIOR SEGMENT CONDITIONS
There have been 7 reported cases of acute corneal graft rejection, occurring between 4 days to 3 weeks following COVID-19 vaccine administration, in the peer-reviewed literature (Table 1).35, 36, 37, 38, 39, 40 Four of the cases occurred in patients with penetrating keratoplasty (PKP), while the 3 remaining patients had Descemet membrane endothelial keratoplasty. Two of the cases with PKP were regrafts, with 1 patient having late endothelial failure following the first PKP graft for keratoconus, while the other patient had a previous failed Descemet stripping automated endothelial keratoplasty for Fuchs endothelial dystrophy, and no other pre-existing risk factors for corneal graft rejection were documented. Six of the 7 cases of graft rejection were reported following recombinant mRNA BNT162b2 (Pfizer/BioNTech) vaccine administration, while the remaining case occurred after adenovirus vector ChAdOx1 nCoV-19 (AstraZeneca) vaccine administration. With the exception of 1 case being reported following the second vaccine dose, graft rejection occurred after the first dose in all 6 remaining patients. Favorable treatment outcomes with resolution of the rejection episode and prevention of graft failure were achieved by timely commencement of intensive topical and/or systemic corticosteroid therapy in all 6 cases with recorded clinical follow-up.
It is acknowledged that causality and direct association cannot be inferred on the basis of case reports, and the potential for coincidental occurrence cannot be completely excluded. However, the close temporal association and lack of other precipitating factors in all 7 cases warrant judicious interpretation. The potential pathophysiological mechanisms underlying vaccine-associated corneal graft rejection remains poorly understood, although rare cases of acute rejection have also previously been reported to occur following hepatitis B, influenza, yellow fever, and tetanus toxoid vaccination.41, 42, 43 Approved COVID-19 vaccines have generally been shown to elicit strong adaptive humoral and cellular immune reactions, including high levels of neutralizing antibody titers and Th1-type CD4+ lymphocytic responses.44 In particular, Th1-type CD4+ lymphocytes have been demonstrated to be among the primary mediators of corneal graft rejection;45 however, it remains uncertain whether the development of cellular antigen cross-reactivity or nonspecific immune activation might have contributed to the reported cases of vaccine-associated rejection.36 , 39
Data from population-based adverse event reporting systems show that post-vaccination corneal graft rejection rates were ≤0.1 cases per million doses (Table 2).18, 19, 20, 21, 22 However, it is acknowledged that these databases did not record the total number of corneal graft recipients that had received vaccination doses, and that the calculation of prevalence rates were conducted using the total number of vaccination doses administered in the general population. Nevertheless, the low overall population prevalence rates would appear to be consistent with the limited number of case reports in the peer-reviewed literature, although the potential influence of reporting and publication bias is acknowledged.
Corneal graft recipients should therefore not be deterred from receiving COVID-19 vaccines, particularly given that the overwhelming benefits will likely outweigh the relatively low risk of graft rejection. Moreover, favorable treatment outcomes were achieved with timely commencement of topical or systemic corticosteroid therapy in all reported cases of vaccine-associated graft rejection with documented clinical follow-up.35, 36, 37 , 39 Currently, there is insufficient evidence to suggest the need for delaying non-urgent corneal graft operations following vaccination administration. Nevertheless, it would remain prudent for eye care practitioners to counsel graft recipients to seek prompt review for ocular discomfort or visual decline following vaccination. In addition, the potential for under-reporting of vaccine-associated graft rejection in the peer-reviewed literature and population-based pharmacovigilance systems cannot be completely excluded. Although there is currently no evidence to support the efficacy of routine prophylactic uptitration of topical corticosteroid therapy in preventing vaccine-associated corneal graft rejection, these treatment measures might be prudently considered in high-risk corneal transplant patients undergoing COVID-19 immunization.46
COVID-19 VACCINATION AND RETINAL CONDITIONS
A few cases of post-vaccination retinal disease have been reported in the peer-reviewed literature, including 6 cases of acute macular neuroretinopathy, 1 case of paracentral acute middle maculopathy, and 1 case of subretinal fluid thought to represent forme fruste central serous chorioretinopathy (Table 1).29 , 47, 48, 49 All cases were documented to occur within 3 to 15 days following immunization. Four of the acute macular neuroretinopathy cases developed following administration of the adenovirus vector ChAdOx1 nCoV-19 (AstraZeneca) vaccine, while the remaining cases of macular pathology occurred following administration of the inactivated virus BBIBP-CorV (Sinopharm) vaccine. Clinical follow-up details were provided for one of the cases with acute macular neuroretinopathy, and the optical coherence tomographic lesions were reported to resolve spontaneously at 2 months. Nevertheless, it was acknowledged that causality and association could not be reliably determined in the context of the typically idiopathic nature of acute macular neuroretinopathy,47 and further clinical details were not provided for the cases presenting with post-vaccination paracentral acute middle maculopathy and subretinal fluid. There have been no reported cases of COVID-19 vaccine-associated adverse events in patients with age-related macular degeneration in the peer-reviewed literature to date.
A single isolated case of acute zonal occult outer retinopathy was reported to occur 10 days following receiving the second dose of the recombinant mRNA-1273 (Moderna) vaccine.50 The patient was treated with an intravitreal dexamethasone implant, and combination azathioprine and cyclosporine therapy was considered, although further clinical follow-up details were not documented. It remains unclear whether cross-reactivity of the vaccination-generated neutralizing antibodies or Th1-type CD4+ lymphocytic response to the proteins and antigens in the outer retinal layers and retinal pigment epithelium might have contributed in the setting of the close temporal association.50
Population-based adverse event reporting systems show that vaccination-associated retinal conditions are rare, with occurrence rates being 0.3 cases per million doses or less (Table 2).18, 19, 20, 21, 22 The low population prevalence rates and isolated case reports in the peer-reviewed literature would suggest that there is insufficient evidence to indicate the requirement for prophylactic treatment or delaying immunization in patients with pre-existing retinal conditions at this juncture.
COVID-19 VACCINATION AND OPTIC NEUROPATHIES
Two cases of COVID-19 vaccine-associated optic neuritis have been reported in the peer-reviewed literature to date (Table 1).51 , 52 The first case occurred in a patient with longstanding relapsing-remittent multiple sclerosis, who developed severe bilateral optic neuritis with chiasmal involvement and longitudinal extensive transverse myelitis, resembling neuromyelitis optica spectrum disorder, 2 weeks following receiving the first dose of the adenovirus vector ChAdOx1 nCoV-19 (AstraZeneca) vaccine.51 The patient had previously been treated with natalizumab for 10 years, with the last dose received 8 days before immunization. Prior to receiving the vaccination, the patient had not previously demonstrated clinical or radiological evidence of neuromyelitis optica spectrum disorder or optic neuritis. Subsequent neuronal autoantibody testing was negative for aquaporin-4, myelin oligodendrocyte glycoprotein (MOG), glial fibrillary acid protein, and flotillin. The patient was treated with high-dose systemic corticosteroids, plasmapheresis, and immunoadsorption, and achieved mild improvement in visual acuities with recognition of motion but not objects. However, there was persistence of paraplegia, loss of sensory function below T5, and incontinence at 2 months. Overall, the onset of symptoms 2 weeks following immunization could potentially be consistent with a dysimmunological process, although it remains uncertain whether this might have been partially attributed to the intended B lymphocyte response targeting the adenovirus vector vaccine, or further exacerbated by the activation and accumulation of B lymphocytes in the peripheral blood incited by natalizumab.51 , 53
The second case occurred in a patient who developed MOG antibody-associated bilateral anterior optic neuritis and concurrent subacute thyroiditis, with symptom onset 6 weeks following the first dose and 12 hours after receiving the second dose of the inactivated virus CoronaVac (Sinovac) vaccine.52 The patient was examined 5 days following symptom onset and demonstrated bilateral optic disc swelling, positive anti-MOG IgG, elevated thyroid stimulating hormone, anti-thyroglobulin, anti-thyroid peroxidase, but normal thyroxine levels. Magnetic resonance imaging (MRI) and lumbar puncture assessment ruled out intracranial hypertension, tumor, and demyelinating disease. The patient was treated with 5 days of intravenous methylprednisolone followed by oral corticosteroid taper, and demonstrated improvements in optic disc swelling, recovery of visual acuity and fields to baseline levels, and normalization of thyroid function within 1 week. In the absence of previous anti-MOG IgG testing, it is difficult to distinguish whether the case represented vaccine-associated or coincidental first manifestation of MOG antibody disorder. However, the possibility of vaccine-associated disease is not inconceivable, with a previous reported case of transient anti-MOG IgG seropositivity following multiple vaccinations for tetanus toxoid, MMR, and varicella zoster,54 as well as a number of cases of concurrent MOG antibody-associated optic neuritis with COVID-19 infection.55, 56, 57
In addition, temporal artery biopsy-positive bilateral arteritic anterior ischemic optic neuropathy (AAION) was reported to occur in a single case, with symptom onset occurring 2 days following the second dose of the recombinant mRNA BNT162b2 (Pfizer/ BioNTech) vaccine.50 The patient had no previous personal or family history of autoimmune disease, and presented for medical review 35 days following initial vision loss. High-dose oral corticosteroid therapy and subcutaneous tocilizumab therapy was commenced, although further clinical follow-up was not documented. The possibility of coincidental disease cannot be discounted, and causation and association cannot be solely inferred on the absence of other precipitating factors. However, the close temporal association between immunization and the onset of autoimmune disease might lend limited support towards the potential contribution of cross-reactivity of the vaccine-generated neutralizing antibodies and/or the Th1-type CD4+ lymphocytic responses towards antigens and proteins expressed in large arteries.50 , 58 Moreover, a previous case of giant cell arteritis was also reported to occur with close temporal association to the administration of the influenza vaccine.58
There have been no reported cases, in the peer-reviewed literature to date, of adverse effects associated with COVID-19 vaccination in patients with glaucoma. Population-based pharmacovigilance reporting shows that post-vaccination optic nerve disorders are relatively rare, with occurrence rates of 1 case per million doses or less (Table 2).18, 19, 20, 21, 22 Overall, in the context of 3 isolated cases of vaccine-associated optic neuropathies in the peer-reviewed literature, as well as the low reported population-based prevalence rates, there is insufficient evidence to mandate prophylactic therapy or delaying immunization in patients with existing optic nerve disorders, although counselling to seek prompt medical attention for visual decline following vaccination would be prudent.
COVID-19 VACCINATION AND OTHER OPHTHALMIC CONDITIONS
Two cases of COVID-19 vaccine-induced immune thrombotic thrombocytopenia with superior ophthalmic vein thrombosis have been reported to date.59 , 60 Both cases occurred 10 days following administration of the first dose of the adenovirus vector ChAdOx1 nCoV-19 (AstraZeneca) vaccine, and in patients with no previous history of autoimmune or thromboembolic conditions. In the first case, the patient presented with severe headache, orbital swelling, proptosis, reduced visual acuity, limited ocular motility, and diplopia. Investigations demonstrated thrombocytopenia, elevated D-dimer levels, and high antibody levels against the platelet factor 4/polyanion complexes, and contrast-enhanced MRI revealed a left superior ophthalmic vein thrombosis. The patient was treated with a 2-day course of intravenous immunoglobulin followed by a tapering course of oral corticosteroid therapy, and commenced on rivaroxaban anticoagulation. Symptomatic resolution occurred within 5 days, platelet levels normalized within 1 week, and D-dimer levels normalized within 2 weeks. In the second case, the patient presented with conjunctival injection, retro-orbital pain, and diplopia. Laboratory investigations showed thrombocytopenia with high antibody levels against the platelet factor 4/polyanion complexes, while contrast-enhance MRI demonstrated bilateral superior ophthalmic vein thrombosis. The patient was treated with intravenous dexamethasone and heparin, but subsequently developed a left parietal lobe ischemic stroke 8 days following admission. The underlying causes of vaccine-induced immune thrombotic thrombocytopenia and secondary thrombosis is not fully understood, but is thought to involve the generation of neoantigens by the binding of vaccine components with platelet factor 4.61, 62, 63, 64 Antibodies are then generated against these neoantigens that can induce pancellular activation, involving platelets, coagulation pathways, monocytes, neutrophils, and endothelial cells, which contributes to significantly elevated thrombotic risk.61, 62, 63, 64
A single case of isolated abducens nerve palsy was reported to occur in a patient 2 days after receiving the recombinant mRNA BNT162b2 (Pfizer/ BioNTech) vaccine.65 The report did not specify whether this was the first or second vaccination dose. An MRI with gadolinium of the brain and orbits demonstrated no cause, and subsequent follow-up clinical details were not provided. Although it is acknowledged that causality cannot be inferred on the basis of a single case report, the potential for vaccine-associated abducens nerve palsy is not inconceivable, with previous cases being reported to occur following the administration of the diphtheria-pertussis-tetanus, MMR, hepatitis B, and influenza vaccines.66, 67, 68, 69, 70 The pathophysiological mechanisms underlying post-vaccination ocular nerve palsies remains unknown, but have been hypothesized to involve immune-mediated demyelination or localized vasculitis.66, 67, 68, 69, 70
There have been 6 case reports in the peer-reviewed literature of Bell's palsy occurring between 2 days to 4 weeks following COVID-19 vaccine administration (Table 1), including 4 cases of unilateral Bell's palsy and 2 cases of bilateral sequential Bell's palsy.71, 72, 73, 74, 75, 76 Three of the 6 cases were reported following recombinant mRNA BNT162b2 (Pfizer/BioNTech) vaccine administration, and the other 3 cases occurred following mRNA-1273 (Moderna) vaccine administration. Two of the cases occurred following the first vaccine dose, and 2 cases occurred following the second dose. In 1 patient with bilateral sequential Bell's palsy, the 2 episodes occurred following the first and second vaccine doses, respectively. Dose details were not available for the remaining 2 patients. Full resolution or significant clinical improvement with steroid therapy were reported in all cases where follow-up details were provided.
The potential association between COVD-19 vaccination and Bell's palsy has been further investigated in a number of observational studies. One retrospective cross-sectional study surveyed participants regarding adverse events occurring following 2 doses of the BNT162b2 (Pfizer/BioNTech) vaccine, and 3 of 299 (1%) participants reported Bell's palsy following the first dose, while 0 of 156 (0%) participants reported Bell's palsy following the second dose.77 The study concluded that Bell's palsy was a rare adverse event, although the absence of a control group, lack of reporting of timing post-vaccination, and the potential for recall bias were acknowledged. Another study reported that 21 of 37 patients (56.8%) admitted for Bell's palsy during a 2-month period had been administered the first or second dose of the BNT162b2 (Pfizer/BioNTech) vaccine within the previous 4 weeks.78 The study also recruited 74 age- and sex-matched controls who were admitted to the same emergency department for other reasons, of which 44 (59.5%) had received a BNT162b2 (Pfizer/BioNTech) vaccine dose within the past 4 weeks. Following adjustment for previous history of peripheral nerve palsy, autoimmune disease, and diabetes, the multivariate OR for the exposure to the vaccine among cases was not statistically significant (OR, 0.84; 95% CI 0.37 to 1.90; P = .67), although it is noted that the study was limited by the modest sample size and potential for selection bias secondary to the hospital-based convenience sample. A large population-based nested case-control study reported that the age-standardized incidence of Bell's palsy was 66.9 cases per 100,000 person-years within 42 days following the inactivated virus CoronaVac (Sinovac) vaccine, and 428 per 100 000 person-years (19.4 to 66.1) within 42 days following the BNT162b2 vaccination.79 The adjusted OR for Bell's palsy was significant for CoronaVac (Sinovac) vaccine relative to the background population (OR, 2.385; 95% CI, 1.415 to 4.022) but not for the BNT162b2 (Pfizer/BioNTech) vaccine (OR, 1.755; 95% CI, 0.886 to 3.477). Nevertheless, it is acknowledged that the observational design of the study precludes the inference of causality. Moreover, the pathophysiology of post-vaccination Bell's palsy remains unclear, and hypothesized mechanisms include molecular mimicry, immune-mediated segmental demyelination, reactivation of latent herpes simplex type 1 infections in the geniculate ganglion, and/or bystander activation of dormant autoreactive T-cells.79 , 80
In conclusion, there has been limited research evaluating the potential ocular adverse effects of COVID-19 vaccines. A small number of case reports have documented the development of uveitis flares and acute corneal graft rejection within the first 3 weeks post-immunization. In addition, rare instances of post-vaccination optic neuropathies, retinal conditions, scleritis, and herpetic eye disease have also been highlighted, although it is acknowledged that causation and direct association cannot be inferred from isolated cases. Overall, data from population-based pharmacovigilance reporting systems would suggest that ocular side effects are very rare following COVID-19 vaccination, and there is no substantive evidence to counterweigh the overwhelming benefits of immunization in patients with pre-existing ophthalmic conditions. However, it is acknowledged that it remains prudent to provide patient counselling to seek prompt medical review for symptoms of post-vaccination visual decline or disease relapse.
Acknowledgments
Funding/Support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Financial disclosures: The authors have no conflicts of interest to declare. All authors attest that they meet the current ICMJE criteria for authorship.
Author contributions: M.T.M.W.: Conceptualization; data curation; methodology; formal analysis; software; writing - original draft. R.L.N.: Conceptualization; writing - review & editing. C.N.J.M.: Conceptualization; writing - review & editing. H.V.D-M.: Conceptualization; methodology; writing - review & editing.
Footnotes
Supplemental Material available at AJO.com.
Appendix. Supplementary materials
REFERENCES
- 1.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus disease 2019 (COVID-19): A review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 2.Ritchie H, Mathieu E, L R-G, et al. University of Oxford; Oxford: 2020. Coronavirus pandemic (COVID-19). Our world in data. Accessed Aug 31, 2021 https://ourworldindataorg/coronavirus. [Google Scholar]
- 3.Woolf SH, Masters RK, LY Aron. Effect of the Covid-19 pandemic in 2020 on life expectancy across populations in the USA and other high income countries: simulations of provisional mortality data. BMJ. 2021;373:n1343. doi: 10.1136/bmj.n1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396:1595–1606. doi: 10.1016/S0140-6736(20)32137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KRW, Pollard AJ. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis. 2021;21:e26–e35. doi: 10.1016/S1473-3099(20)30773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Creech CB, Walker SC, Samuels RJ. SARS-CoV-2 vaccines. JAMA. 2021;325:1318–1320. doi: 10.1001/jama.2021.3199. [DOI] [PubMed] [Google Scholar]
- 7.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein NP, Lewis N, Goddard K, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021 doi: 10.1001/jama.2021.15072. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kochhar S, Salmon DA. Planning for COVID-19 vaccines safety surveillance. Vaccine. 2020;38:6194–6198. doi: 10.1016/j.vaccine.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hippisley-Cox J, Patone M, Mei XW, et al. Risk of thrombocytopenia and thromboembolism after Covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374:n1931. doi: 10.1136/bmj.n1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021 doi: 10.1001/jama.2021.13443. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thakur KT, Tamborska A, Wood GK, et al. Clinical review of cerebral venous thrombosis in the context of COVID-19 vaccinations: Evaluation, management, and scientific questions. J Neurol Sci. 2021;427 doi: 10.1016/j.jns.2021.117532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng XL, Betzler BK, Testi I, et al. Ocular adverse events after COVID-19 vaccination. Ocul Immunol Inflamm. 2021 doi: 10.1080/09273948.2021.1976221. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization (WHO) World Health Organization (WHO) Collaborating Centre for International Drug Monitoring; Upsala: 2020. Programme for International Drug Monitoring.https://www.ho-umcorg/global-pharmacovigilance/who-programme-for-international-drug-monitoring/ Accessed Jul 12, 2021. [Google Scholar]
- 18.Database of Adverse Event Notifications (DAEN). Canberra: Therapeutic Goods Administration (TGA), Australian Government Department of Health; 2021. Accessed Aug 31, 2021. https://www.tgagovau/database-adverse-event-notifications-daen
- 19.Canada Vigilance adverse reaction database. Ottawa: Health Canada; 2021. Accessed Aug 31, 2021. https://www.canadaca/en/health-canada/services/drugs-health-products/medeffect-canada/adverse-reaction-databasehtml
- 20.European Medicines Agency, European Union; Amsterdam: 2021. EudraVigilance: European database of suspected adverse drug reaction.https://www.adrreportseu/ Accessed Aug 31, 2021. [Google Scholar]
- 21.Coronavirus (COVID-19) vaccine adverse reactions. London: Medicines and Healthcare Products Regulatory Agency, United Kingdom Department of Health and Social Care; 2021. Accessed Aug 31, 2021. https://www.govuk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions
- 22.Vaccine Adverse Event Reporting System (VAERS). Atlanta: Centers for Disease Control and Prevention, United States Department of Health and Human Services; 2021. Accessed Aug 31, 2021. https://wondercdcgov/vaershtml
- 23.Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220647. in press. [DOI] [PubMed] [Google Scholar]
- 24.ElSheikh RH, Haseeb A, Eleiwa TK, Elhusseiny AM. Acute uveitis following COVID-19 vaccination. Ocul Immunol Inflamm. 2021 doi: 10.1080/09273948.2021.1962917. in press. [DOI] [PubMed] [Google Scholar]
- 25.Goyal M, Murthy SI, Annum S. Bilateral multifocal choroiditis following COVID-19 vaccination. Ocul Immunol Inflamm. 2021 doi: 10.1080/09273948.2021.1957123. in press. [DOI] [PubMed] [Google Scholar]
- 26.Rabinovitch T, Ben-Arie-Weintrob Y, Hareuveni-Blum T, et al. Uveitis following the BNT162b2 mRNA vaccination against SARS-CoV-2 infection: a possible association. Retina. 2021 doi: 10.1097/iae.0000000000003277. in press. [DOI] [PubMed] [Google Scholar]
- 27.Mudie LI, Zick JD, Dacey MS, Palestine AG. Panuveitis following vaccination for COVID-19. Ocul Immunol Inflamm. 2021;29:741–742. doi: 10.1080/09273948.2021.1949478. [DOI] [PubMed] [Google Scholar]
- 28.Papasavvas I, Herbort CP., Jr. Reactivation of Vogt-Koyanagi-Harada disease under control for more than 6 years, following anti-SARS-CoV-2 vaccination. J Ophthalmic Inflamm Infect. 2021;11:21. doi: 10.1186/s12348-021-00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pichi F, Aljneibi S, Neri P, Hay S, Dackiw C, Ghazi NG. Association of ocular adverse events with inactivated COVID-19 vaccination in patients in Abu Dhabi. JAMA Ophthalmol. 2021 doi: 10.1001/jamaophthalmol.2021.3477. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benage M, Fraunfelder FW. Vaccine-associated uveitis. Mo Med. 2016;113:48–52. [PMC free article] [PubMed] [Google Scholar]
- 31.Furer V, Zisman D, Kibari A, Rimar D, Paran Y, Elkayam O. Herpes zoster following BNT162b2 mRNA Covid-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford) 2021 doi: 10.1093/rheumatology/keab345. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thimmanagari K, Veeraballi S, Roach D, Al Omour B, Slim J. Ipsilateral zoster ophthalmicus post COVID-19 vaccine in healthy young adults. Cureus. 2021;13:e16725. doi: 10.7759/cureus.16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter R, Hartmann K, Fleisch F, Reinhart WH, Kuhn M. Reactivation of herpes virus infections after vaccinations? Lancet. 1999;353:810. doi: 10.1016/S0140-6736(99)00623-6. [DOI] [PubMed] [Google Scholar]
- 34.Richardson-May J, Rothwell A, Rashid M. Reactivation of herpes simplex keratitis following vaccination for COVID-19. BMJ Case Reports. 2021;14 doi: 10.1136/bcr-2021-245792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crnej A, Khoueir Z, Cherfan G, Saad A. Acute corneal endothelial graft rejection following COVID-19 vaccination. J Fr Ophtalmol. 2021 doi: 10.1016/j.jfo.2021.06.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phylactou M, Li JO, Larkin DFP. Characteristics of endothelial corneal transplant rejection following immunisation with SARS-CoV-2 messenger RNA vaccine. Br J Ophthalmol. 2021;105:893–896. doi: 10.1136/bjophthalmol-2021-319338. [DOI] [PubMed] [Google Scholar]
- 37.Rallis KI, Ting DSJ, Said DG, Dua HS. Corneal graft rejection following COVID-19 vaccine. Eye (Lond) 2021 doi: 10.1038/s41433-021-01671-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ravichandran S, Natarajan R. Corneal graft rejection after COVID-19 vaccination. Indian J Ophthalmol. 2021;69:1953–1954. doi: 10.4103/ijo.IJO_1028_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wasser LM, Roditi E, Zadok D, Berkowitz L, Weill Y. Keratoplasty rejection after the BNT162b2 messenger RNA vaccine. Cornea. 2021;40:1070–1072. doi: 10.1097/ICO.0000000000002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Renisi G, Lombardi A, Stanzione M, Invernizzi A, Bandera A, Gori A. Anterior uveitis onset after bnt162b2 vaccination: is this just a coincidence? Int J Infect Dis. 2021;110:95–97. doi: 10.1016/j.ijid.2021.07.035. [DOI] [PubMed] [Google Scholar]
- 41.Vignapiano R, Vicchio L, Favuzza E, Cennamo M, Mencucci R. Corneal graft rejection after yellow fever vaccine: a case report. Ocul Immunol Inflamm. 2021 doi: 10.1080/09273948.2020.1870146. in press. [DOI] [PubMed] [Google Scholar]
- 42.Steinemann TL, Koffler BH, Jennings CD. Corneal allograft rejection following immunization. Am J Ophthalmol. 1988;106:575–578. doi: 10.1016/0002-9394(88)90588-0. [DOI] [PubMed] [Google Scholar]
- 43.Wertheim MS, Keel M, Cook SD, Tole DM. Corneal transplant rejection following influenza vaccination. Br J Ophthalmol. 2006;90:925. doi: 10.1136/bjo.2006.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 45.Amouzegar A, Chauhan SK, Dana R. Alloimmunity and tolerance in corneal transplantation. J Immunol. 2016;196:3983–3991. doi: 10.4049/jimmunol.1600251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lockington D, Lee B, Jeng BH, Larkin DFP, Hjortdal J. Survey of corneal surgeons' attitudes regarding keratoplasty rejection risk associated with vaccinations. Cornea. 2021 doi: 10.1097/ICO.0000000000002662. in press. [DOI] [PubMed] [Google Scholar]
- 47.Book BAJ, Schmidt B, Foerster AMH. Bilateral acute macular neuroretinopathy after vaccination against SARS-CoV-2. JAMA Ophthalmol. 2021;139 doi: 10.1001/jamaophthalmol.2021.2471. [DOI] [PubMed] [Google Scholar]
- 48.Bøhler AD, Strøm ME, Sandvig KU, Moe MC, Jørstad ØK. Acute macular neuroretinopathy following COVID-19 vaccination. Eye (Lond) 2021 doi: 10.1038/s41433-021-01610-1. in pess. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mambretti M, Huemer J, Torregrossa G, Ullrich M, Findl O, Casalino G. Acute macular neuroretinopathy following Coronavirus disease 2019 vaccination. Ocul Immunol Inflamm. 2021;29:730–733. doi: 10.1080/09273948.2021.1946567. [DOI] [PubMed] [Google Scholar]
- 50.Maleki A, Look-Why S, Manhapra A, Foster CS. COVID-19 recombinant mRNA vaccines and serious ocular inflammatory side effects: real or coincidence? J Ophthalmic Vis Res. 2021;16:490–501. doi: 10.18502/jovr.v16i3.9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Helmchen C, Buttler GM, Markewitz R, Hummel K, Wiendl H, Boppel T. Acute bilateral optic/chiasm neuritis with longitudinal extensive transverse myelitis in longstanding stable multiple sclerosis following vector-based vaccination against the SARS-CoV-2. J Neurol. 2021 doi: 10.1007/s00415-021-10647-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leber HM, Sant'Ana L, Konichi da Silva NR, et al. Acute thyroiditis and bilateral optic neuritis following SARS-CoV-2 vaccination with CoronaVac: a case report. Ocul Immunol Inflamm. 2021 doi: 10.1080/09273948.2021.1961815. in press. [DOI] [PubMed] [Google Scholar]
- 53.Traub J, Häusser-Kinzel S, Weber MS. Differential effects of MS therapeutics on B cells - implications for their use and failure in AQP4-positive NMOSD patients. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21145021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar N, Graven K, Joseph NI, et al. Case report: postvaccination anti-myelin oligodendrocyte glycoprotein neuromyelitis optica spectrum disorder: a case report and literature review of postvaccination demyelination. Int J MS Care. 2020;22:85–90. doi: 10.7224/1537-2073.2018-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sawalha K, Adeodokun S, Kamoga GR. COVID-19-induced acute bilateral optic neuritis. J Investig Med High Impact Case Rep. 2020;8 doi: 10.1177/2324709620976018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kogure C, Kikushima W, Fukuda Y, et al. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis in a COVID-19 patient: A case report. Medicine (Baltimore) 2021;100:e25865. doi: 10.1097/MD.0000000000025865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Žorić L, Rajović-Mrkić I, Čolak E, Mirić D, Kisić B. Optic neuritis in a patient with seropositive myelin oligodendrocyte glycoprotein antibody during the post-COVID-19 period. Int Med Case Rep J. 2021;14:349–355. doi: 10.2147/IMCRJ.S315103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liozon E, Parreau S, Filloux M, et al. Giant cell arteritis or polymyalgia rheumatica after influenza vaccination: A study of 12 patients and a literature review. Autoimmun Rev. 2021;20 doi: 10.1016/j.autrev.2020.102732. [DOI] [PubMed] [Google Scholar]
- 59.Panovska-Stavridis I, Pivkova-Veljanovska A, Trajkova S, Lazarevska M, Grozdanova A, Filipche V. A rare case of superior ophthalmic vein thrombosis and thrombocytopenia following ChAdOx1 nCoV-19 vaccine against SARS-CoV-2. Mediterr J Hematol Infect Dis. 2021;13 doi: 10.4084/MJHID.2021.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bayas A, Menacher M, Christ M, Behrens L, Rank A, Naumann M. Bilateral superior ophthalmic vein thrombosis, ischaemic stroke, and immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination. Lancet (London, England) 2021;397:e11. doi: 10.1016/S0140-6736(21)00872-2. -e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Althaus K, Möller P, Uzun G, et al. Antibody-mediated procoagulant platelets in SARS-CoV-2-vaccination associated immune thrombotic thrombocytopenia. Haematologica. 2021;106:2170–2179. doi: 10.3324/haematol.2021.279000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huynh A, Kelton JG, Arnold DM, Daka M, Nazy I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature. 2021;596:565–569. doi: 10.1038/s41586-021-03744-4. [DOI] [PubMed] [Google Scholar]
- 65.Reyes-Capo DP, Stevens SM, Cavuoto KM. Acute abducens nerve palsy following COVID-19 vaccination. JAAPOS. 2021;25:302–303. doi: 10.1016/j.jaapos.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Woo EJ, Winiecki SK, Ou AC. Motor palsies of cranial nerves (excluding VII) after vaccination: reports to the US Vaccine Adverse Event Reporting System. Hum Vaccin Immunother. 2014;10:301–305. doi: 10.4161/hv.27032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Werner DB, Savino PJ, Schatz NJ. Benign recurrent sixth nerve palsies in childhood. Secondary to immunization or viral illness. Arch Ophthalmol. 1983;101:607–608. doi: 10.1001/archopht.1983.01040010607016. [DOI] [PubMed] [Google Scholar]
- 68.Bourtoulamaiou A, Yadav S, Nayak H. Benign Recurrent sixth (abducens) nerve palsy following measles-mumps-rubella vaccination. Case Rep Pediatr. 2015;2015 doi: 10.1155/2015/734516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leiderman YI, Lessell S, Cestari DM. Recurrent isolated sixth nerve palsy after consecutive annual influenza vaccinations in a child. JAAPOS. 2009;13:317–318. doi: 10.1016/j.jaapos.2008.12.137. [DOI] [PubMed] [Google Scholar]
- 70.Grewal DS, Zeid JL. Isolated abducens nerve palsy following neonatal hepatitis B vaccination. JAAPOS. 2014;18:75–76. doi: 10.1016/j.jaapos.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 71.Burrows A, Bartholomew T, Rudd J, Walker D. Sequential contralateral facial nerve palsies following COVID-19 vaccination first and second doses. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-243829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Colella G, Orlandi M, Cirillo N. Bell's palsy following COVID-19 vaccination. J Neurol. 2021;268:3589–3591. doi: 10.1007/s00415-021-10462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iftikhar H, Noor SMU, Masood M, Bashir K. Bell's palsy after 24 hours of mRNA-1273 SARS-CoV-2 vaccine. Cureus. 2021;13:e15935. doi: 10.7759/cureus.15935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mason MC, Liaqat A, Morrow J, Basso R, Gujrati Y. Bilateral facial nerve palsy and COVID-19 vaccination: causation or coincidence? Cureus. 2021;13:e17602. doi: 10.7759/cureus.17602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martin-Villares C, Vazquez-Feito A, Gonzalez-Gimeno MJ, de la Nogal-Fernandez B. Bell's palsy following a single dose of mRNA SARS-CoV-2 vaccine: a case report. J Neurol. 2021 doi: 10.1007/s00415-021-10617-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Repajic M, Lai XL, Xu P, Liu A. Bell's palsy after second dose of Pfizer COVID-19 vaccination in a patient with history of recurrent Bell's palsy. Brain Behav Immun Health. 2021;13 doi: 10.1016/j.bbih.2021.100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.El-Shitany NA, Harakeh S, Badr-Eldin SM, et al. Minor to moderate side effects of Pfizer-BioNTech COVID-19 vaccine among Saudi residents: a retrospective cross-sectional study. Int J Gen Med. 2021;14:1389–1401. doi: 10.2147/IJGM.S310497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shemer A, Pras E, Einan-Lifshitz A, Dubinsky-Pertzov B, Hecht I. Association of COVID-19 vaccination and facial nerve palsy: a case-control study. JAMA Otolaryngol Head Neck Surg. 2021;147:739–743. doi: 10.1001/jamaoto.2021.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wan EYF, Chui CSL, Lai FTT, et al. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Principi N, Esposito S. Do vaccines have a role as a cause of autoimmune neurological syndromes? Front Public Health. 2020;8:361. doi: 10.3389/fpubh.2020.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.