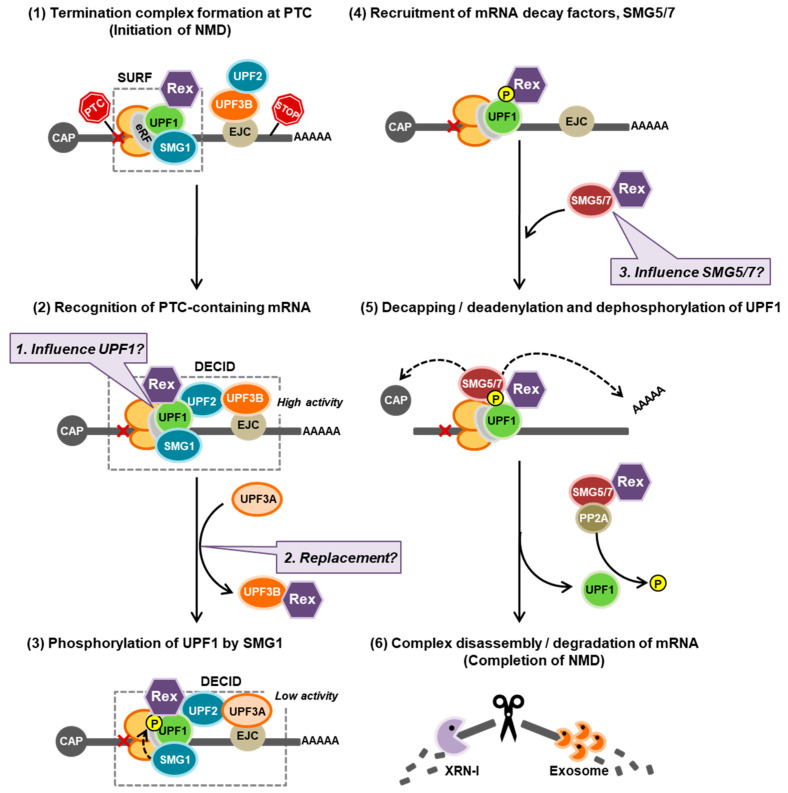
Figure 8.
Possible model of Rex-NMD complex interaction and new questions. NMD progresses in a cascade as shown in (1–6). (1): Our results suggest that Rex may inhibit NMD by intervening from the beginning to the end of this cascade by interacting with the NMD key regulator Upf1 and may have some effect on its activity (Baloon-1). (2): Rex interacts only with Upf3B, suggesting that Rex substitutes Upf3A for Upf3B in the NMD complex, resulting in the inclusion of less active Upf3A in the NMD complex, thereby suppressing NMD activity (Baloon-2). (4,5): The mRNA decay factors, the SMG5/7 complex, are recruited to phosphorylated Upf1. The SMG5/7 complex recruits the decapping complex (DCP2/DCP1a) and the deadenylation complex CCR4–NOT to enhance decapping and deadenylation of the NMD target mRNA. Additionally, the SMG5/7 complex recruits protein phosphatase 2A (PP2A) for dephosphorylation of Upf1. Since dephosphorylation of Upf1 is essential for NMD completion, Rex may influence the dephosphorylation pathway of Upf1 via SMG5/7 interaction, and thus may influence NMD completion (Baloon-3).