Abstract
The manufacturing of sanitary and household furniture on a large scale with inherently antimicrobial properties is an essential field of research. This work focuses on the synthesis of polymer composites based on the unsaturated polyester of resin loaded with 5 wt.%-Triclosan produced by a co-mixing approach on automated technological complex with a potential for broad applications. According to findings, the polymer composite has a non-porous structure (surface area < 1.97 m2/g) suitable for sanitary applications to reduce the growth of bacteria. The chemical composition confirmed the presence of major elements, and the inclusion of Triclosan was quantitatively confirmed by the appearance of chlorine on XRF (1.67 wt.%) and EDS (1.62 wt.%) analysis. Thermal analysis showed the difference of 5 wt.% in weight loss, which confirms the loading of Triclosan into the polymer matrix. The polymer composite completely inhibited the strains of S. aureus 6538-P, S. aureus 39, S. epidermidis 12228, and Kl. Pneumoniae 10031 after 5-min contact time. The antimicrobial effects against Kl. pneumoniae 700603, Ps. aeruginosa 9027 and Ps. aeruginosa TA2 strains were 92.7%, 85.8% and 18.4%, respectively. The inhibition activity against C. albicans 10231 and C. albicans 2091 was 1.6% and 82.4%, respectively; while the clinical strain of C. albicans was inhibited by 92.2%. The polymer composite loaded with 5 wt.%-Triclosan displayed a stability over the period that illustrates the possibility of washing the composite surface.
Keywords: polymer, composite resin, antimicrobial activity, triclosan, antibacterial
1. Introduction
According to the World Health Organization, dangerous microbes such as bacteria, viruses, and parasites in food cause diarrheal diseases, which make 550 million people sick and cause 230,000 deaths each year, while inadequate drinking water, sanitation, and handwashing practices cause 842,000 diarrheal deaths in low- and middle-income countries. The development of microbial resistance to antibiotics further complicates the situation [1]. Therefore, keeping the sectors of massive contact free of microbial contamination is paramount for the health and safety of people.
Microbial contamination is largely associated with numerous human health-related sectors such as hospitals and dental equipment, food packaging and storage, water treatment systems, kitchen and dining accessories, and household sanitation equipment. The unavoidable presence of harmful microorganisms on the surface of those furniture and daily commodities resulted in the escalation of various infections and diseases. Commonly used surface coatings or backbone materials, such as wood, steel, glass and plastics, could partially “serve” as an environment for the growth and spread of microorganisms [2,3,4]. This might be due to the porous microstructure of those commonly applied porous materials, which allows the penetration of water or moisture to create a potential growth environment for microorganisms [3,4]. Moreover, frequent contact with water or disinfectants due to daily cleaning services could gradually destroy the surface and shorten the duration of kitchen accessories, household and hospital sanitary furniture or wares. To avoid these issues, materials with non-porous structures might be a solution, where either the building block or scaffold itself or the attached bactericidal agent may serve as an inherently antimicrobial material or surface coating for those types of necessities. In addition, the polymeric scaffolds to be used as the backbone material or surface coatings for those applications must have a potential for large-scale manufacturing, as well as to provide materials in different shapes, geometries and sizes to expand the application fields and meet market requirements.
Disinfectants such as hypochlorite, hydrogen peroxide, quaternary ammonium compounds, silver salts, or other reactive oxygen species have been examined for these uses, but their short duration and limited environmental friendliness make their application highly competitive [5]. At the same time, new macromolecules with antimicrobial properties and structural modifications of various materials, including polymers, are being developed to achieve disinfectant properties with desired physicochemical parameters [6]. According to Bonilla et al. [7], antimicrobial materials could be separated into four categories: (a) those that exhibit antimicrobial activity on their own; (b) those for which biocidal activity is obtained by chemical modification; (c) those that include either low or high molecular weight (MW) antimicrobial organic compounds; and (d) those involving the addition of active inorganic systems.
Antimicrobial polymers are a class of materials designed to kill or inhibit the growth of microbes on surfaces or in the environment. Polymers such as chitosan, quaternary nitrogen composites, halamines, and poly-ε-lysine may have inherent antimicrobial properties [8,9], while other polymers act as scaffolds for the immobilization of small biocides and antibiotics to enhance bactericidal activity [10,11,12]. Antimicrobial polymers currently under study include substituted or modified natural polymers, antimicrobial polymers containing multiple biocidal units attached to the backbone, polymers with a satellite terminal biocidal unit, polymer–antibiotic composite, and polymer–inorganic antimicrobial composites [13,14]. Organic (chlorhexidine, triclosan, polyaniline, and polyethyleneimine) agents are commonly used to modify polymers to give them antimicrobial properties. Among these agents, triclosan (2,4,4-trichloro-2-hydroxydiphenyl ether) is widely used in personal care products, such as in toothpaste [15], hand soaps and cosmetics [16], and polymers to prevent the process of degradation and colonization over time [17]. At low concentrations, Triclosan has a bacteriostatic effect, due to the harmful influence of bacterial enzymes responsible for the composition of fatty acids from the cell wall and membrane, while at high concentrations, triclosan destroys the bacterial membrane, leading to its death [18]. Due to its broad antimicrobial spectrum, it is used in commercial resin sealants [19], in halloy–site nanotubes for composite resins [20,21], and has also been encapsulated in nanocapsules for addition to adhesive resin [22], demonstrating good physical and chemical properties without cytotoxicity. However, in studies by Genari et al. [22], it was found that after 120 h, almost 20% of triclosan is released from the adhesive resin network, which might be due to the porous structure of the substrate. Polymeric scaffolds with higher drug retention can reduce the leaching of triclosan and keep its chemical structure available for interaction with bacteria for a prolonged duration. Particularly, the inherently non-porous polymer composites might be a solution, as they entrap the attached antibacterial drugs or agents within the structure of the matrix. Table 1 summarizes a number of related research works on biomedical and antimicrobial applications, where synthetic and natural polymers, as well as polymeric composites employed as support matrices for organic or inorganic antimicrobial agents. However, most of these polymeric substrates have a porous structure to some extent or are limited in processing for small scales only, which may potentially reduce their market application for the manufacturing and production of daily sanitary and household accessories and furniture.
Table 1.
Selected research on polymer and composite materials loaded with antimicrobial agents.
| Title | Support Matrix | Antimicrobial Agent | Antimicrobial Activity Target | Ref. |
|---|---|---|---|---|
| Triclosan-loaded chitosan as antibacterial agent for adhesive resin | Chitosan | Triclosan | Streptococcus mutans | [23] |
| Preparation and release study of Triclosan in polyethylene/Triclosan anti-bacterial blend | Polyethylene | Triclosan | Escherichia coli; Klebsiella pneumoniae; Staphylococcus aureus | [24] |
| Poly(ε-caprolactone)/Triclosan loaded polylactic acid nanoparticles composite: a longterm antibacterial bionanocomposite with sustained release | Poly(ε-caprolactone)/polylactic acid nanoparticles | Triclosan |
Staphylococcus aureus; Escherichia coli |
[25] |
| Antimicrobial polymer composites with copper micro- and nanoparticles: effect of particle size and polymer matrix |
Polypropylene; polyamide 6; high-density polyethylene | Copper NPs |
Staphylococcus aureus; Pseudomonas aeruginosa |
[26] |
| Investigations of antibacterial activity of chitosan in the polymeric composite coatings |
Chitosan | Acrylic resin | Staphylococcus aureus, | [27] |
| Structural, thermal, and antibacterial properties of chitosan/ZnO composites |
Chitosan | Zinc oxide |
Pseudomonas aeruginosa; Escherichia coli; Staphylococcus aureus |
[28] |
| Antibacterial effect of composite resins containing quaternary ammonium polyethyleneimine nanoparticles |
Restorative composite resin | Quaternary ammonium polyethyleneimine |
Streptococcus mutans | [29] |
| Preparation of chitosan-g-poly(acrylamide)/montmorillonite superabsorbent polymer composites: studies on swelling, thermal, and antibacterial properties |
Chitosan-g- poly(acrylamide)/montmorillonite | Chitosan |
Staphylococcus aureus; Escherichia coli |
[30] |
| Polyethylene/silver-nanofiber composites: a material for antibacterial films |
Polyethylene | Silver nanofiber | Escherichia coli | [31] |
| A novel antibacterial resin composite for improved dental restoratives |
Composite resin | Functional furanone derivative | Streptococcus mutans | [32] |
| Structural, thermal and antibacterial properties of polyamide 11/polymeric biocide polyhexamethylene guanidine dodecylbenzenesulfonate composites |
Polyamide 11/polymeric biocide polyhexamethylene guanidine |
Dodecylbenzenesulfonate |
Escherichia coli; Bacillus subtilis |
[33] |
| Synthesis and characterization of a novel biodegradable antimicrobial polymer |
Polyurethane (1,6-hexane diisocyanate, polycaprolactone diol) |
Ciprofloxacin (fluoroquinolone antibiotic) | Pseudomonas aeruginosa | [34] |
| Dual-functional antifogging/antimicrobial polymer coating | Poly(2-(dimethylamino)- ethyl methacrylate-co-methyl methacrylate) and polymerized ethylene glycol dimethacrylate network |
Quaternary ammonium compound | Staphylococcus epidermidis; Escherichia coli | [35] |
| Antifouling and antimicrobial polymer membranes based on bioinspired polydopamine and strong hydrogen-bonded poly(N-vinyl pyrrolidone) |
Polypropylene coated with a polydopamine layer and modified by poly(N-vinyl pyrrolidone) | Iodine | Staphylococcus aureus | [36] |
| Nitric oxide-loaded antimicrobial polymer for the synergistic eradication of bacterial biofilm |
Polymer of oligoethylene glycol, hydrophobic ethylhexyl and cationic primary amine | Nitric oxide | Pseudomonas aeruginosa | [37] |
| Dual-mechanism antimicrobial polymer–ZnO nanoparticle and crystal violet-encapsulated silicone |
Medical grade silicone | Di(octyl)- phosphinic acid capped ZnO nanoparticles |
Staphylococcus aureus; Escherichia coli |
[11] |
| Novel antimicrobial polymer films active against bacteria and fungi |
Polypropylene and linear low-density polyethylene | 4′-hydroxy-(4- hydroxy-3-ethyl)-azobenzene (azo-dye) |
Staphilococcus aureus; Candida albicans |
[38] |
This research work focuses on the synthesis of polymeric composites based on unsaturated polyester resin attached to the organic antimicrobial agent triclosan into non-porous structure by a co-mixing approach on the advanced complex, which allows the manufacturing of polymer composite-based sanitary and household furniture on a large scale. The samples of obtained pristine and triclosan-loaded polymeric composites with antimicrobial properties are characterized on cutting-edge techniques such as XRF, SEM-EDS, CHNS, BET and TGA to retrieve chemical, morphological and thermal characteristics that are essential for the proposed applications. The current research apprehended a broad range of strains of bacteria and fungi that commonly exists on various surfaces in comparison to previous studies. This allows one to effusively evaluate the antimicrobial activity of the pristine and triclosan-loaded polymeric composites. In addition, the mechanical properties of the polymeric composites, antimicrobial performance over several cycles of re-use along with a release of the loaded antimicrobial agent triclosan, have been explored and meticulously discussed.
2. Materials and Methods
2.1. Materials
The following chemical reagents are used to produce polymer composites with and without loading the antimicrobial agent: unsaturated polyester of orthophthalic acid (99%), methyl ethyl ketone peroxide (99%) and triclosan (5-chloro-2-(2,4-dichloro-phenoxy)phenol) (99.5 wt.%), CaCO3 (95 wt.%), lecithin (Phosphatidylcholine: min. 60%, Escherichia coli: negative, Iodine number: 60–70; Peroxide number: max. 3.0; Lysophosphatidylcholine: max. 3%; non-polar lipids: max. 20%; Water (K.F.): max. 2%) and tween-80 1310 g/mol were purchased from AppliChem, Darmstadt, Germany. The synthesis of polymer composites with and without loading triclosan was triplicated and average synthesis characteristics are presented. Culture media and reagents nutrient broth, nutrient agar, and tryptic soy broth were purchased from Himedia, Mumbai, India. All chemical reagents were of analytical grade and used as received without prior purification or treatment.
(1) Staphylococcus aureus ATCC 6538-P—a sensitive test strain obtained from the Republican collection of microorganisms (RCM), Nur-Sultan, RK;
(2) Staphylococcus aureus ATCC BAA-39—a resistant test strain obtained from the American Type Culture Collection (ATCC), Manassas, VA, USA;
(3) Staphylococcus epidermidis ATCC 12228—a sensitive test strain obtained from the American Type Culture Collection (ATCC), Manassas, VA, USA;
(4) Klebsiella pneumoniae ATCC 10031—a resistant test strain obtained from the American Type Culture Collection (ATCC), Manassas, VA, USA;
(5) Klebsiella pneumoniae ATCC 700603—a sensitive test strain obtained from the American Type Culture Collection (ATCC), Manassas, VA, USA;
(6) Pseudomonas aeruginosa 9027—a sensitive test strain obtained from the American Type Culture Collection (ATCC), Manassas, VA, USA;
(7) Pseudomonas aeruginosa TA2—a resistant clinical isolate;
(8) Candida albicans ATCC 10231—a test strain obtained from the American Type Culture Collection (ATCC), Manassas, VA, USA;
(9) Candida albicans ATCC 2091—test strain obtained from the American Type Culture Collection (ATCC), Manassas, VA, USA;
(10) Candida albicans—a clinical isolate.
2.2. Synthesis of Polymer Composites with Triclosan
To produce a composite material, the required amounts of unsaturated polyester resin, filler (CaCO3), and additive (Triclosan) were thoroughly mixed in a vacuum mixer until a viscous homogeneous mass was obtained. The ratio of reagents used in the synthesis of polymer composite is shown in Table 2. The presented data show the composition of the reference and experimental samples of polymer composites produced in this research. The experimental sample includes the addition of antimicrobial agent triclosan (5 wt.%), while the reference sample is excluded.
Table 2.
Production of polymer composite loaded with triclosan.
| Sample Name | Composition of Samples | Weight % |
|---|---|---|
| Reference polymer composite | Unsaturated polyester resin | 28 |
| Calcium carbonate | 70 | |
| Methyl ethyl ketone peroxide (MEKP) | 2 | |
| Triclosan loaded polymer composite | Unsaturated polyester resin | 28 |
| Calcium carbonate | 65 | |
| Methyl ethyl ketone peroxide (MEKP) | 2 | |
| Triclosan | 5 |
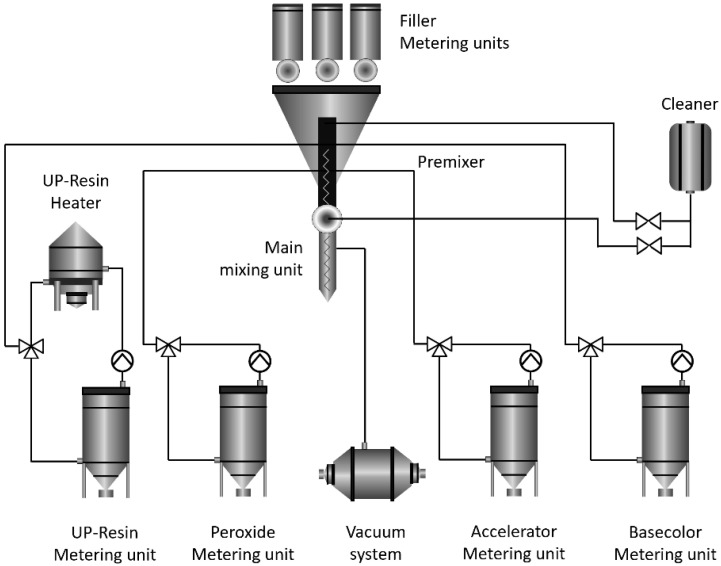
In order to synthesize a non-porous polymeric composite, the liquid substances of the composition unsaturated polyester resins of orthophthalic acid and methyl ethyl ketone peroxide were quickly and vigorously mixed in a preliminary mixer (premixer, RESPECTA, Wulfrath, Germany) with a capacity of at least 2800 rpm within 2 s to avoid the development of bubbles. This is due to the fact that, after the addition of peroxide into the resin, the mixture becomes highly reactive. After this, the mixed liquid substances were further vigorously mixed under 1400 rpm for 5–6 s in the main mixer unit (RESPECTA, Wulfrath, Germany) with the addition of solid components (CaCO3 and triclosan). During mixing, a vacuum pump with a power of at least 0.1 bar was used to evacuate air from the mixture. Next, the mixture was poured into special molds, while eliminate the inlet of air from the external environment and inside the mold itself. This was avoided by using closed molds by forcing air out of the mold when the mixture was poured. The rate of the polymerization (hardening) of the material controlled the ambient temperature.
As the catalyst is added, the resin becomes more viscous, until it reaches a state where it is no longer a liquid and is no longer able to flow. This is the state of termination of polymerization. The resin continues to harden after it has thickened until it reaches its full hardness over time. This reaction itself is accompanied by the release of heat due to an exothermic reaction, which, in turn, accelerates the reaction. The temperature at the peak reaches over 50 °C. The whole process is known as “curing” the resin, which lasts for about 1 h and 20 min. Further details of the synthesis of polymeric composite from unsaturated polyester resin of orthophthalic acid, as well as the alternative synthesis of aromatic copolyesters of biosourced-phthalic acids (dimethoxyterephthalate), tiophene–aromatic polyesters could be found elsewhere [14,39,40]. Figure 1 shows the schematic diagram of the polymer composite production process on an automated technological complex.
Figure 1.
Preparation of polymer composite on automated technological complex.
2.3. Characterization of Polymer Composite
A chemical analysis of polymer composites was conducted using a standardless X-ray fluorescence (XRF, Malvern Panalytical, Cambridge, UK) spectrometer under helium atmosphere in a powder sample mode. For XRF analysis, a tablet was prepared from the following ratio: 5 g of polymer composite and 1.2 g of boric acid additive. The two powders were thoroughly mixed in an agate mortar for 20 min. Then, the fine powder was placed in a steel round mold and placed under a vacuum press at a pressure of at least 20 atm. After 30 min, the tablet was removed and placed in a special plastic sample holder for research. The sample holder was placed in an XRF analyzer and examined for elements in a helium atmosphere. Total carbon, hydrogen, nitrogen, and sulfur elemental analysis was carried out using a vario El Cube (Elementar Analysensysteme, Langenselbold, Germany) CHNS analyzer.
The thermal properties of polymer composite samples were identified using a Simultaneous Thermal Analyzer STA6000 (Perkin Elmer, Waltham, MA, USA). For analysis, about 10 g of a sample was taken and placed in special heat-resistant crucibles. The samples were heated in an atmosphere of pure nitrogen from 50 °C to 700 °C at a heating rate of 10 °C/min to reveal the thermal stability of the polymer composite and measure the amount of inorganic residues (ash) formed after high-temperature combustion.
The morphological characteristics of the polymer composite with and without triclosan were studied on scanning electron microscopy (SEM) using a JEOL 6380LV Scanning Electron Microscope (JEOL, Tokyo, Japan), operating in LV mode, at 20 KV, equipped with a backscattered electron detector. Spot and area analyses were carried out using a Si(Li) Energy-Dispersive X-ray spectrometer (INCA X-sight, Oxford Instruments, Abingdon, UK), connected to SEM.
The porous structure was determined using low-temperature nitrogen adsorption measured on an Autosorb-1 porosimeter (Quantochrome, Hook, UK). The average pore size and total pore volume were calculated from the experimental data obtained from porosimetric studies using the in-built software Asiqwin. The specific surface area was calculated using the BET model. For a functional groups analysis, FT-IR spectra of the samples in the form of fine powder were recorded in the range of 4000–400 cm−1 with a resolution of 4 cm−1 using a Cary 600 Series FTIR spectrophotometer (Agilent Technologies, Santa Clara, CA, USA) equipped with an ATR module.
2.4. Antimicrobial Activity Tests of Polymer Composite
The antimicrobial testing parameters using the pristine and Triclosan-loaded polymer compoistes are shown in Table 3.
Table 3.
The parameters used in the biological studies.
| Surface Type of Sample | Chemical Composition | ||||
|---|---|---|---|---|---|
| Sample | calcium carbonate, unsaturated ester of orthophthalic acid, methyl ethyl ketone peroxide, triclosan content 5 wt.%; 8 cm in diameter | ||||
| Reference sample | calcium carbonate, unsaturated ester of orthophthalic acid, methyl ethyl ketone peroxide without triclosan; 8 cm in diameter | ||||
| Release liner size | 40 mm × 40 mm | ||||
| Test strains cultivation Medium | Nutrient agar, pH 7.4 ± 0.2 | Incubation time and conditions | 37 ± 1 °C; 18–24 h |
||
| Inoculum preparation medium | 1/250 Nutrient broth, pH 7.4 ± 0.2 | Inoculum concentration | 2.5–10.0 × 105 CFU/mL | Amount of applied inoculum | 0.4 mL |
| Contact time and incubation conditions | 37 ± 1 °C; Humidity: ≥90%, 2 h | ||||
| Neutralizer | Tryptic soy broth with lecithin and tween-80, pH 6.8–7.2 | ||||
| Medium for counting CFU | Nutrient agar, pH 7.4 ± 0.2 | Incubation time and conditions | 37 ± 1 °C; 40–48 h |
||
2.4.1. Preparation of Test Samples
Before the study, the control and experimental composite samples were placed in individual sterile Petri dishes. At the same time, polyethylene release liner was prepared, the size of which was 40 × 40 (±2.0) mm. Previously, all samples, as well as the release liner, were sterilized with 70% ethanol. After testing each strain, the composite samples were also sterilized with 70% ethanol.
2.4.2. Preparation of Suspensions of Test Strains
Notably, 18–24 h’s culture of test strains were used in the study. In a test tube containing 5–6 mL 1/250 nutrient broth; an aliquot of the corresponding test strain was added with a sterile loop and homogenized. The optical density was measured densitometrically, which was 1.2 units, according to McFarland, which corresponded to a cell concentration of 3.6 × 108 CFU/mL for bacterial suspensions and 3.6 × 106 CFU/mL for yeast cells. The working concentration of cells was prepared by 10-fold serial dilutions of the stock suspension until a final inoculum of 3.6 × 105 CFU/mL was obtained for each test strain.
2.4.3. Inoculation
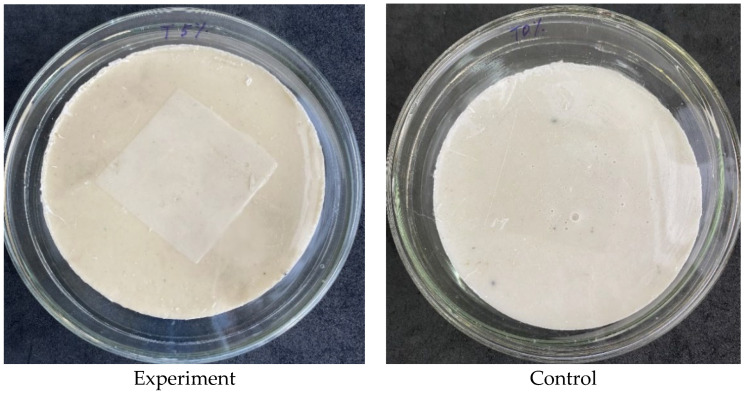
On the surface of the experimental and control samples, 0.4 mL of the corresponding strain suspension was added. To evenly distribute the inoculum over the surface, the samples were immediately covered with a release liner (Figure 2), after the test samples were placed in a thermostat at 37 °C for two hours of incubation.
Figure 2.
Inoculated experimental (with 5 wt.% Triclosan) and control (without Triclosan) composite samples covered with the release liner.
2.4.4. Washing Samples with a Neutralizing Agent
After two hours of incubation, the control and experimental samples were washed with a neutralizer as tryptic soy broth with lecithin (1 g/L) and tween-80 (7 g/L) as nontoxic surfactants. Washing was carried out as follows: 10 mL of a neutralizer was added to the Petri dish containing the sample, and the culture was removed from the sample surface, carefully pipetting the contents of the dish.
Note: The use of a neutralizing agent is necessary to avoid false positive results of the antimicrobial activity presence, because the residual amount of the active substance contained in the samples can, to some extent, reduce the number of viable cells.
2.4.5. Inoculation and Counting Colony Forming Units (CFU)
To count CFU, a series of 10-fold serial dilutions (1/10, 1/100) was prepared from the washings of each sample, after which deep inoculation was performed from the washings and prepared dilutions (from one dilution to 2 PE). The amount of inoculum was 1 mL. The inoculated plates were flooded with a warm agar medium (46–48 °C) in a volume of 15–20 mL and the contents were gently mixed. After the solidification of the medium, the dishes were placed in a thermostat. After the incubation time, a direct count of the grown CFUs was performed visually.
2.4.6. Number Determination of the Viable Bacteria
For each sample, the definition of viable bacteria was calculated according to Equation (1):
| N = (100 × C × D × V)/A | (1) |
where,
N is the number of CFU/sample;
C is the average number of CFU on duplicated plates;
D is the dilution factor;
V is the volume of the used neutralizer, mL;
A is the area of the release liner, mm2
All experiments were designed and conducted according to standard protocols JIS Z 2801:2000 ISO 22196:2007, ASTM E 1054 [41].
3. Results and Discussion
3.1. Synthesis of Polymer Composite with Triclosan
The results of the elemental analysis show that the framework of the polymer composite has a carbonate structure, where the content of calcium, as the main mineral, is about 49%, and the remaining elements of the framework of the polymer composite are magnesium, sulfur, iron, oxygen, and carbon. The polymer composite with 5 wt.%-triclosan, in turn, revealed a successful modification with the antibacterial agent triclosan, where 1.67% chlorine is detected, the molecule of which contains three atoms of chlorine (Table 4). These results prove that the polymer composite has been successfully modified with the triclosan and the method are suitable for the synthesis of antibacterial polymer composites.
Table 4.
Chemical composition of polymer composites (%).
| Chemical Elements | Content in Polymer Composite | Polymer Composite with 5 wt.%-Triclosan |
|---|---|---|
| Mg | 0.40 | 0.39 |
| Ca | 48.99 | 47.61 |
| S | 0.02 | 0.02 |
| Fe | 0.01 | 0.01 |
| Cl | - | 1.67 |
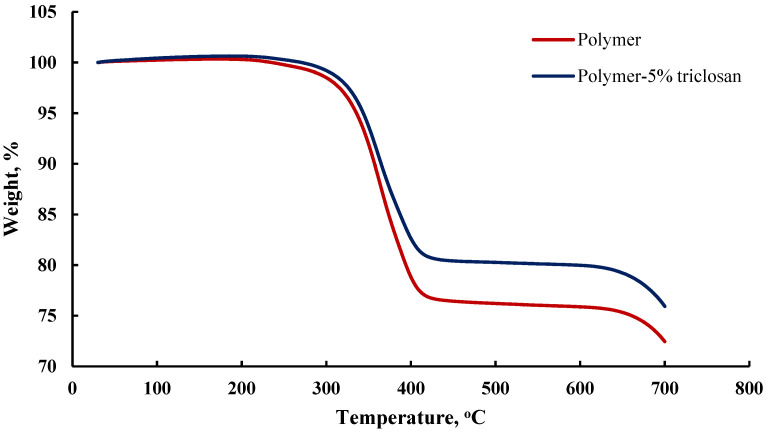
It can be concluded that the polymer composite with triclosan has a greater organic component than the polymer composite. It was found that the mass fraction of the combustion residues of the polymer composite after heat treatment in nitrogen flow is about 26%, and for the polymer composite with triclosan, it is about 31%, due to the aromatic nature of the molecule, which also participated in the carbonization process (Figure 3). Moreover, we can confidently assume that the matrix of the polymer composite has a non-porous structure, since, in the case of porous materials, a step of weight loss of adsorbed water should have appeared, due to adsorbed and hygroscopic water in the structure of the material.
Figure 3.
Thermogravimetric analysis of the pristine and 5 wt.%-triclosan-loaded polymer composites at heating rate of 10 °C/min from 50 °C to 700 °C.
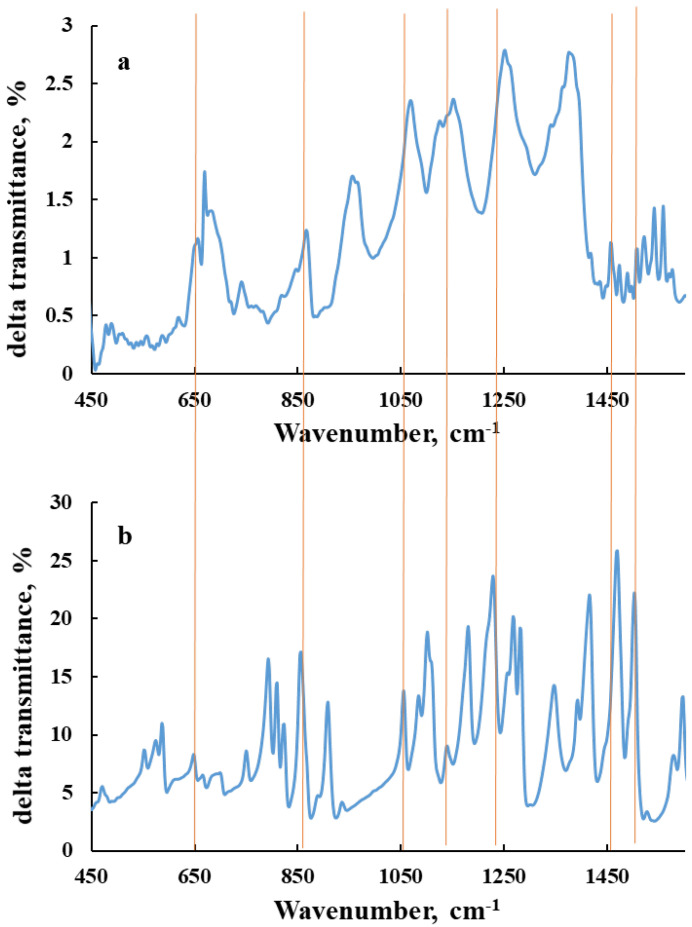
The FTIR spectra of polymer composite and 5 wt.%-loaded triclosan polymer composite are presented in Figure 4. Since both samples are made from polymeric precursors, it is very difficult to differentiate the functional groups of triclosan from the polymeric matrix. The peaks at 742 and 871 cm−1 are attributed to the =C–H aromatic vibration, and we have polyaromatic polymers that overlay with characteristic bonds of =C–H of triclosan. According to the literature, the high intensity vibration bond for Triclosan is registered at 1485 cm−1 Bojar et al. [42]. The frequency C–Cl cannot be detected due to the low content of triclosan in the composition, and due to the average and low intensity of the bond.
Figure 4.
FT-IR spectra of the 5 wt.%-triclosan-loaded polymer composite (a) and triclosan (b).
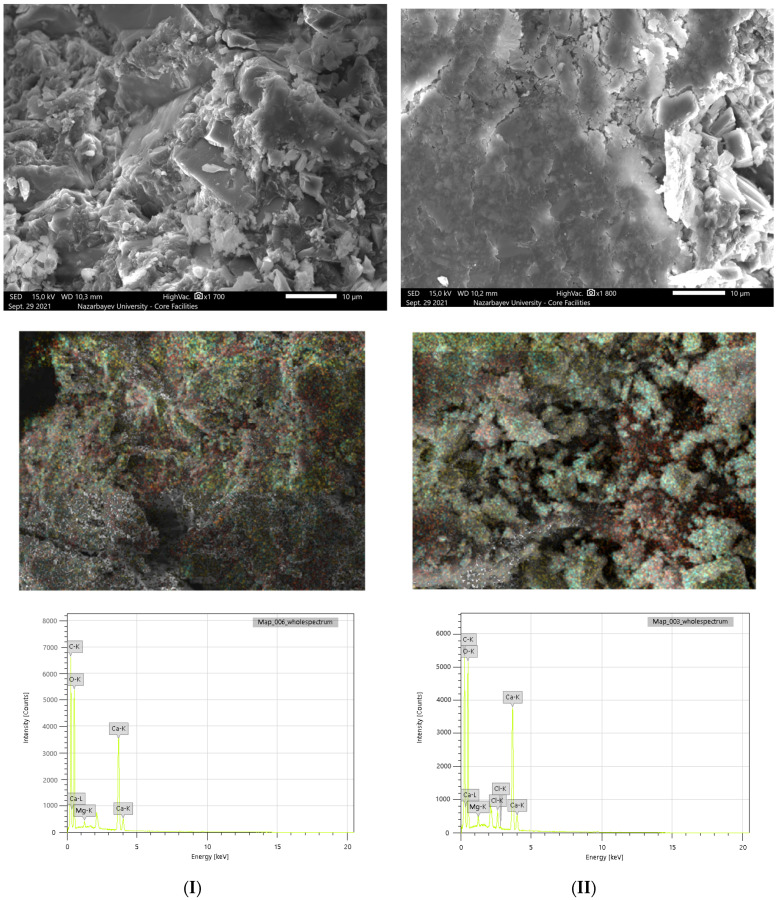
The morphological analysis of the original and modified polymer composite with the antibacterial agent triclosan shows the non-porous structure of the samples. These morphological characteristics together with mechanical properties are favorable for the production of sanitary and construction products based on a polymer composite, in order to effectively prevent the spread of microorganisms. The main advantages of the proposed polymer composite are non-porosity (monolithic material without pores) and high maintainability. Due to its non-porosity, microorganisms have less probability to grow on the surface of the material. Therefore, it allows them to be applied as sanitary products and as building materials. In the event of defects on the surface of the material, they can be easily eliminated by sanding and polishing the defective spots. The results of the SEM study of the polymer composite are shown in Figure 5, while the mechanical properties can be found in the Supplementary Materials, Section 1.
Figure 5.
SEM-EDS micrographs of the pristine (I) and 5 wt.%-triclosan-loaded (II) polymer composites.
Samples of the original and modified polymer composite were also analyzed by the quantitative EDS method, which shows the content of the main elements in the selected area of the SEM micrographs. The mass fractions of calcium and magnesium in the polymer composite are 21.87% and 0.56%, respectively (Table 5). The EDS analysis illustrated that the modified composite, in addition to these metals, revealed 1.92% chlorine, confirming the successful impregnation of triclosan into the matrix of the material, which most probably takes place due to p–p stucking. These results are consistent with those obtained on an X-ray fluorescence analysis, which in turn confirms the validity of the results.
Table 5.
The elemental analysis of the polymeric composites on SEM-EDS (in wt.%).
| Sample | Ca | Mg | C | O | Cl |
|---|---|---|---|---|---|
| Polymer composite | 21.87 | 0.56 | 32.00 | 45.57 | - |
| Polymer composite with 5 wt.%-triclosan | 22.89 | 0.77 | 29.97 | 44.45 | 1.92 |
The original polymer composites and polymer composites with the antibacterial agent triclosan (5 wt.%) were studied on a CHNS elemental microanalyzer in duplicate, which allows one to calculate the exact amounts of carbon (C), hydrogen (H), nitrogen (N), and sulfur (S).
The main element in the framework of the samples is carbon, which varies from 24.49 wt.% to 25.95 wt.% (Table 6). Notably, there was a decrease in the mass fraction of nitrogen and sulfur, which is associated with the addition of 5 wt.% of the antibacterial agent tricolsan; each molecule contains three atoms of chlorine. The data obtained are comparable to the results of X-ray fluorescence analysis (XRF) and with an elemental analysis of a scanning electron microscope (SEM-EDS), which also revealed carbon, hydrogen, nitrogen, and sulfur in an approximate mass fraction.
Table 6.
The microelemental analysis of polymeric composites on a CHNS analyzer (in wt.%).
| Sample | C | H | N | S |
|---|---|---|---|---|
| Polymer composite | 25.95 ± 0.34 | 1.14 ± 0.15 | 4.99 ± 0.71 | 0.32 ± 0.05 |
| Polymer composite with 5 wt.%-triclosan | 24.49 ± 0.52 | 1.24 ± 0.02 | 2.36 ± 0.45 | 0.28 ± 0.01 |
A porosimetric analysis of the pristine and modified polymer composite with 5 wt.%-triclosan shows the insignificant specific surface area for both samples. The specific surface areas of the composite and the modified composite with triclosan were 1.964 m2/g and 0 m2/g, respectively. A decrease in the specific surface area of the modified polymer composite can be associated with the addition of an antibacterial agent, as a result of which, the structure becomes even denser, and the number of pores is significantly reduced. This is essentially important, as a potential application of the proposed polymer composite with triclosan is the manufacturing of large-scale sanitary and household furniture, where the entrapment of any water or moisture may create an environment for the growth of microorganisms. The pore sizes of the polymer composites, calculated by the BJH method, exhibit a non-porous structure, where the pore radius of the samples is about 17 ± 0.11 Å.
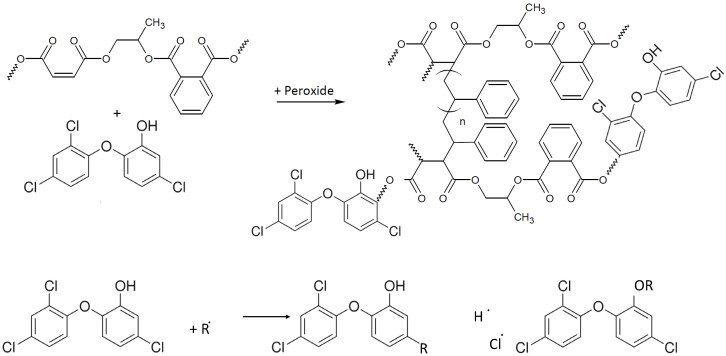
A kinetic of release of triclosan from the polymeric composite to water was carried out at room temperature at moderate shaking (160 rpm). No significant increase of the absorbance at 282 nm was observed after five days (Figure S1). From the obtained results, one can conclude that most triclosan is covalently linked to the polymer backbone or it is strongly physically adsorbed on the nonporous polymeric composite via hydrophobic interactions. Moreover, triclosan is a very lipophilic substance with solubility in water at only 10 ppm. It is probable that the accelerated diffusion of triclosan takes place via the lipophilic contact of the cell’s membrane with the composite surface. This finding of triclosan release is very attractive from a practical point of view. It illustrates the possibility of washing the composite surface by pure water with the minimal leakage of the antimicrobial agent, and therefore prolonged usage maintaining a bactericidal effect. Based on the results obtained and the performance of the polymer composite loaded with 5 wt.%-triclosan, the synthetic reaction of polymerization is presented in Figure 6.
Figure 6.
The proposed synthetic reaction of the polymer with triclosan.
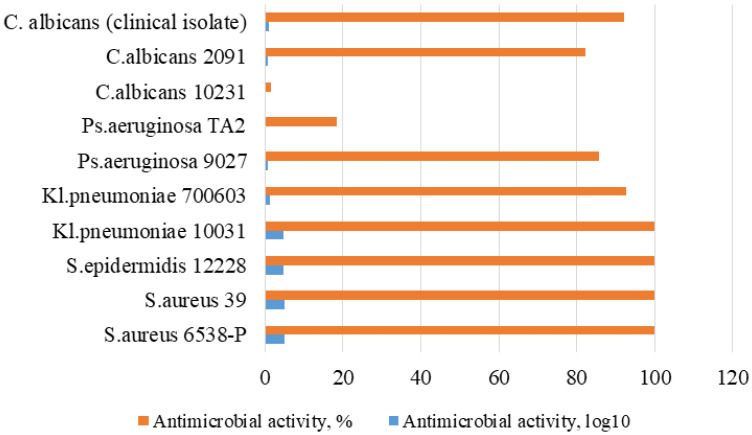
3.2. Antimicrobial Activity of Polymer Composite with Triclosan
The study of the polymeric composite antimicrobial substance triclosan (5 wt.%) was carried out against eight strains of Gram-positive and Gram-negative bacteria at 2-h exposure by direct contact of viable cells of microorganisms with the sample. An identical composite sample without triclosan was used as a negative control. All manipulations with the experimental and control samples were carried out in parallel under the same conditions. The results of antimicrobial activity testing are presented in Table 7 and Figure 7; a comparative graphical interpretation is shown in Figure 8. From previous reports about polymeric composites with triclosan for various composites, we expected that the developed system will illustrate a bacteriostatic effect [43].
Table 7.
Results of dynamics of antimicrobial activity of the polymer composite with triclosan.
| Strain | Contact Time | Sample | Average CFU | Average log10 | Antimicrobial Activity, log10 | Antimicrobial Activity,% |
|---|---|---|---|---|---|---|
| S. aureus 6538-P | 5 min | negative control | 7.50 × 103 | 3.875 | 5.079 | 100 |
| composite triclosan | 0.06 | −1.204 | ||||
| S. aureus 39 | 5 min | negative control | 6.38 × 103 | 3.804 | 5.009 | 100 |
| composite triclosan | 0.06 | −1.204 | ||||
| S. epidermidis 12228 | 5 min | negative control | 4.16 × 103 | 3.619 | 4.823 | 100 |
| composite triclosan | 0.06 | −1.204 | ||||
| Kl. pneumoniae 10031 | 5 min | negative control | 2.91 × 103 | 3.463 | 4.667 | 100 |
| composite triclosan | 0.06 | −1.204 | ||||
| Kl. pneumoniae 700603 | 5 min | negative control | 8.88 × 103 | 3.948 | 0.005 | 1.1 |
| composite triclosan | 8.78 × 103 | 3.944 | ||||
| 15 min | negative control | 9.06 × 103 | 3.957 | 0.006 | 1.4 | |
| composite triclosan | 8.94 × 103 | 3.951 | ||||
| 30 min | negative control | 7.19 × 103 | 3.857 | 0.006 | 1.3 | |
| composite triclosan | 7.09 × 103 | 3.851 | ||||
| 1 h | negative control | 7.16 × 103 | 3.855 | 0.006 | 1.3 | |
| composite triclosan | 7.06 × 103 | 3.849 | ||||
| 2 h | negative control | 6.19 × 103 | 3.792 | 0.104 | 21.2 | |
| composite triclosan | 4.88 × 103 | 3.688 | ||||
| 4 h | negative control | 2.05 × 104 | 4.311 | 1.135 | 92.7 | |
| composite triclosan | 1.50 × 103 | 3.176 | ||||
| Ps. aeruginosa 9027 | 5 min | negative control | 8.34 × 103 | 3.921 | 0.002 | 0.4 |
| composite triclosan | 8.31 × 103 | 3.920 | ||||
| 15 min | negative control | 6.66 × 103 | 3.823 | 0.002 | 0.5 | |
| composite triclosan | 6.73 × 103 | 3.821 | ||||
| 30 min | negative control | 6.94 × 103 | 3.841 | 0.004 | 0.9 | |
| composite triclosan | 6.88 × 103 | 3.837 | ||||
| 1 h | negative control | 6.69 × 103 | 8.825 | 0.004 | 0.9 | |
| composite triclosan | 6.63 × 103 | 3.821 | ||||
| 2 h | negative control | 5.72 × 103 | 3.757 | 0.005 | 1.1 | |
| composite triclosan | 5.66 × 103 | 3.753 | ||||
| 4 h | negative control | 3.31 × 103 | 3.520 | 0.849 | 85.8 | |
| composite triclosan | 0.47 × 103 | 2.671 | ||||
| Ps. aeruginosa TA2 | 5 min | negative control | 2.66 × 103 | 3.424 | 0.016 | 3.5 |
| composite triclosan | 2.56 × 103 | 3.409 | ||||
| 15 min | negative control | 2.84 × 103 | 3.454 | 0.020 | 4.4 | |
| composite triclosan | 2.72 × 103 | 3.434 | ||||
| 30 min | negative control | 3.00 × 103 | 3.477 | 0.018 | 4.2 | |
| composite triclosan | 2.88 × 103 | 3.459 | ||||
| 1 h | negative control | 1.66 × 103 | 3.219 | 0.034 | 7.5 | |
| composite triclosan | 1.53 × 103 | 3.185 | ||||
| 2 h | negative control | 1.22 × 103 | 3.086 | 0.086 | 17.9 | |
| composite triclosan | 1.00 × 103 | 3.00 | ||||
| 4 h | Negative control | 1.19 × 103 | 3.075 | 0.088 | 18.4 | |
| Composite triclosan | 0.97 × 103 | 2.986 | ||||
| C. albicans 10231 | 5 min | Negative control | 1.12 × 104 | 4.049 | 0.001 | 0.3 |
| Composite triclosan | 1.12 × 104 | 4.048 | ||||
| 15 min | Negative control | 1.08 × 104 | 4.034 | 0.001 | 0.3 | |
| Composite triclosan | 1.08 × 104 | 4.033 | ||||
| 30 min | Negative control | 9.16 × 103 | 3.962 | 0.001 | 0.3 | |
| Composite triclosan | 9.13 × 103 | 3.960 | ||||
| 1 h | Negative control | 3.38 × 103 | 3.972 | 0.004 | 1.0 | |
| Composite triclosan | 3.97 × 103 | 3.968 | ||||
| 2 h | Negative control | 1.08 × 104 | 4.035 | 0.006 | 1.4 | |
| Composite triclosan | 1.07 × 104 | 4.029 | ||||
| 4 h | Negative control | 9.97 × 103 | 3.999 | 0.007 | 1.6 | |
| Composite triclosan | 9.81 × 103 | 3.992 | ||||
| C. albicans 2091 | 5 min | Negative control | 8.19 × 103 | 3.913 | 0.002 | 0.4 |
| Composite triclosan | 8.16 × 103 | 3.911 | ||||
| 15 min | Negative control | 5.28 × 103 | 3.723 | 0.003 | 0.6 | |
| Composite triclosan | 5.25 × 103 | 3.720 | ||||
| 30 min | Negative control | 5.53 × 103 | 3.743 | 0.002 | 0.6 | |
| Composite triclosan | 5.50 × 103 | 3.740 | ||||
| 1 h | Negative control | 5.12 × 103 | 3.710 | 0.003 | 0.6 | |
| Composite triclosan | 5.09 × 103 | 3.707 | ||||
| 2 h | Negative control | 5.09 × 103 | 3.707 | 0.051 | 11.0 | |
| Composite triclosan | 4.53 × 103 | 3.656 | ||||
| 4 h | Negative control | 3.84 × 103 | 3.585 | 0.747 | 82.1 | |
| Composite triclosan | 0.69 × 103 | 2.837 | ||||
| C. albicans (clinical isolate) | 5 min | Negative control | 8.38 × 103 | 3.923 | 0.002 | 0.4 |
| Composite triclosan | 8.34 × 103 | 3.921 | ||||
| 15 min | Negative control | 8.78 × 103 | 3.944 | 0.002 | 0.4 | |
| Composite triclosan | 8.75 × 103 | 3.942 | ||||
| 30 min | Negative control | 8.63 × 103 | 3.936 | 0.003 | 0.7 | |
| Composite triclosan | 8.56 × 103 | 3.933 | ||||
| 1 h | Negative control | 8.97 × 103 | 3.953 | 0.181 | 34.1 | |
| Composite triclosan | 5.91 × 103 | 3.771 | ||||
| 2 h | Negative control | 1.67 × 104 | 4.222 | 0.352 | 55.5 | |
| Composite triclosan | 7.41 × 103 | 3.870 | ||||
| 4 h | Negative control | 8.84 × 103 | 3.947 | 1.109 | 92.2 | |
| Composite triclosan | 0.69 × 103 | 2.837 |
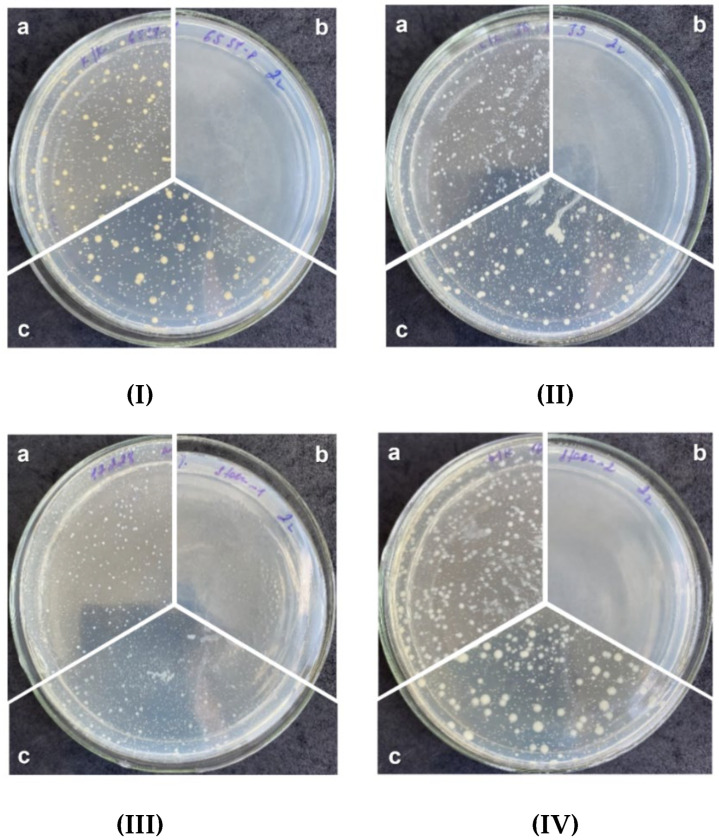
Figure 7.
The results of antimicrobial activity testing of composites against S. aureus 6538-P (I), S. aureus 39 (II), S. epidermidis 12228 (III), and Kl. pneumoniae 10031 (IV): a—negative control; b—Polymer composite with 5 wt.%-Triclosan; c—polymer composite.
Figure 8.
The change in the antimicrobial activity against different strains of microorganisms of the polymer composite with 5 wt.%-triclosan.
The maximum antibacterial effect of Triclosan-containing composite specimens was shown in relation to the strains of S. aureus ATSS VAA-39, S. aureus ATSS 6538-P, and S. epidermidis with aphids, epoxy, epoxy, ATSS, 22x pneumoniae ATSS 10031, which needed only 5 min of contact time with the antimicrobial composite (Figures S2–S4). Some bacteriostatic effect in relation to P. aeruginosa ATSS 9027 and fungicidal activity is noted after four hours of contact time with the composite containing triclosan (Figure S5). Thus, as expected, the composite of calcium carbonate, unsaturated ester of orthophthalic acid, and peroxide methyl ethyl ketone did not reveal any bacteriostatic effect.
From the other side, an addition of triclosan (5 wt.%) illustrated significant inhibition of colony growth in vitro. Minimum inhibitory concentrations (MIC) for some strains were higher than for others, which is why longer incubation time with a polymer was necessary to achieve MIC. A high antimicrobial effect against S. epidermidis ATSS 12228 and Kl. pneumoniae ATSS 10031 at 5 min of contact time (100% cell death) was observed (Table 7). It was reported that the MIC of triclosan against the abovementioned strain is about 1 μg/mL [44], and one can assume that five minutes of incubation provided a diffusion of triclosan which was higher than MIC. The inhibiting activity against Ps. aeruginosa ATSS 9027 was noticed only for four hours of incubation (reduction of growth strain by 85.8%) and these data correlate with previously published data illustrating the insignificant activity of triclosan for a concentration range of 12.5–100 μmol/L [45]. The fungicidal activity was estimated against strains of C. albicans ATSS 2091 and clinical isolate of C. albicans and the reduction of the growth of the strain by 92.2% was at a prolonged contact time of four hours (Table 7). It is worth noting that antimicrobial activity in relation to strains of microorganisms Kl. pneumoniae ATSS 700603, Ps. aeruginosa TA2 and C. albicans ATSS 10231 was not detected (Figures S6–S11). However, according to previous research, the MIC of triclosan for Kl. pneumoniae is quite low—about 0.5–0.8 μg/mL [43]. For C. albicans ATSS 10231, there is no information for triclosan, but the MIC of caffeine is 12.5 μmol/L [46].
Only five mins of incubation of the triclosan-containing composite sample was enough to observe 100% bactericidal activity against the bacteria of the genus Staphylococcus: S. aureus 6538-P, S. aureus 39, S. epidermidis 12228, as well as the sensitive strain Kl. pneumoniae 10031 (Table 7). However, against the resistant strain Kl. pneumoniae 700603, the bactericidal activity is reduced to 92.7%, while for Ps. aeruginosa 9027 and Ps. aeruginosa TA2 strains, it demonstrated bactericidal activity of 85.8% and 18.4%, respectively. The study of fungicidal activity demonstrates a moderate antimicrobial effect against strains C. albicans 10231 and C. albicans 2091, expressed in 1.6% and 82.4%, respectively, after four hours of contact time. These results are comparable with previous studies conducted with triclosan-loaded chitosan as an antibacterial agent for adhesive resin on the strains of S. mutans [23], an antibacterial blend of polyethylene with triclosan on the strains of E. coli and Kl. pneumoniae; S. aureus [24] as well as with the findings on poly(ε-caprolactone)/triclosan-loaded polylactic acid nanoparticles composite examined on the strains of S. aureus and E. coli [25]. The major advantage of the proposed 5 wt.%-triclosan-loaded polymer composite in the current study is the wide range of studied samples (10 strains), which allowed one to broadly assess the antibacterial performance. Moreover, it was observed that polymer composite reveals a high antimicrobial effect on the clinical strain of C. albicans 92.2% (Table 7), which has not been examined on the triclosan-loaded polymer or polymer composite matrix in the literature, to the best of our knowledge.
Thus, the maximum antimicrobial effect of the composite sample with 5 wt.%-triclosan within the studied exposure period was noted against all 10 strains, wherein a complete inhibition was observed for Staphylococcus aureus (sensitive and resistant strains) and epidermal staphylococci and susceptible strain Kl. pneumoniae 10031 (Figure 7).
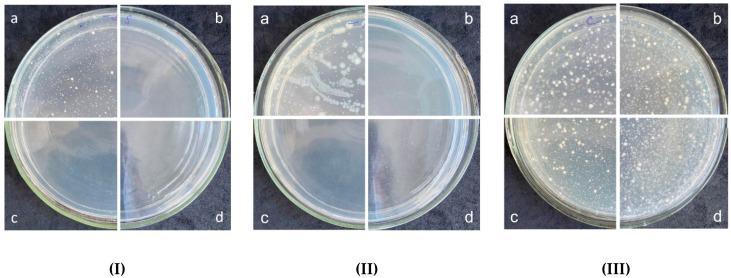
Studies to assess the antimicrobial activity of composites under abrasion conditions were carried out against 2 bacterial strains and 1 yeast strain—S. aureus ATCC 6538-P, Kl. pneumoniae ATCC 10031, C. albicans ATCC 10231, respectively. Testing was carried out for three cycles of abrasion. The contact time for bacterial and yeast strains were 2 h and 4 h, respectively. The results of the experiment are presented in Figure 9. The obtained values indicate the preservation of 100% antimicrobial activity against the strain of S. aureus 6538-P after three cycles of attrition. The bactericidal activity of the samples against the strain Kl. pneumoniae 10031 after three cycles of attrition also remains unchanged. The data obtained when testing the yeast strain C. albicans 10231 demonstrate the absence of fungicidal activity after four hours of contact time with the triple abrasion of the samples.
Figure 9.
The results of antimicrobial activity of the polymer composite with 5 wt.%-Triclosan against the strain of S. aureus 6538-P (I), Kl. pneumoniae 10031 (II), C. albicans 10231 (III) after three cycles of attrition: a—negative control; b—1st cycle; c—2nd cycle; d—3rd cycle.
4. Conclusions
The polymer composite with antibacterial agent triclosan obtained on an automated technological complex allows one to produce surface coating materials for sanitary applications on a large-scale. According to findings, the polymer composite has indeed a non-porous structure that is further improved by loading antibacterial agent triclosan. The incorporation of triclosan was confirmed by XRF and SEM-EDS analyses, and proved to be a successful modification in terms of the presence of chlorine in the modified polymer substrate. Thermal analysis quantitatively confirmed the difference of 5 wt.% of weight loss between pristine and modified polymer composites, which is in corroboration with the initially loaded triclosan amount that increased aromatic content during carbonization. The antimicrobial activity studies of the triclosan-loaded polymer composite displayed a fast and complete antimicrobial effect against S. aureus 6538-P, S. aureus 39 strains, S. epidermidis 12228, and Kl. Pneumoniae 10031. The inhibition performance against remaining strains varied between 1.6% and 92.7%, wherein the antimicrobial activity against a clinical strain of C. albicans was 92.2%. The polymer composite showed steady performance after three cycles of use by showing similar antimicrobial activity. The release profile of loaded triclosan within a polymer matrix showed a high stability for a prolonged period, which enhances its potential application as a non-porous building block for a large-scale production of sanitary and household accessories and furniture.
Acknowledgments
Zhandos Tauanov would like to thank the funding of the postdoctoral fellowship of al-Farabi Kazakh National University by the decree of №4-3650 from 16 September 2020 to the educational program “Nanomaterials and nanotechnology in chemistry” (2020–2023).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/polym14040676/s1, Figure S1: UV-Vis spectrum of the release kinetics of Triclosan from polymer matrix (triplicated), Figure S2: The results of antimicrobial activity of composites against strain S. aureus 6538-P contact time 5 min, Figure S3: The results of antimicrobial activity of composites against strain S. aureus 39 contact time 5 min, Figure S4. The results of antimicrobial activity of composites against strain S. epidermidis 12228 contact time 5 min, Figure S5: The results of antimicrobial activity of composites against strain Ps. aeruginosa 9027, Figure S6: The results of antimicrobial activity of composites against strain Kl. pneumoniae 700603, Figure S7: The results of antimicrobial activity of composites against strain C. albicans 10231, contact time 2 h, Figure S8: The results of antimicrobial activity of composites against strain C. albicans 2091, contact time 2 h, Figure S9: The results of antimicrobial activity of composites against strain C. albicans contact time 2 h, Figure S10: The results of antimicrobial activity of composites against strain Kl. pneumoniae 10031 contact time 5 min, Figure S11: The results of antimicrobial activity of composites against strain Kl. pneumoniae 700603, contact time 2 h.
Author Contributions
Conceptualization, Z.T.; Data curation, Z.T.; Funding acquisition, O.Z.; Investigation, O.Z., Z.B., A.B., N.S.A. and D.B.; Methodology, Z.B.; Resources, O.Z.; Supervision, Z.T.; Validation, A.B.; Visualization, N.S.A.; Writing—original draft, Z.T.; Writing—review & editing, D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan, grant number AP09058298 (2021–2023). The APC was funded The APC was funded from the “The Best Teacher of University 2020 of MES RK” award for Dmitriy Berillo.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lode H.M. Clinical impact of antibiotic-resistant Gram-positive pathogens. Clin. Microbiol. Infect. 2009;15:212–217. doi: 10.1111/j.1469-0691.2009.02738.x. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharjee T., Datta S.S. Bacterial hopping and trapping in porous media. Nat. Commun. 2019;10:2–10. doi: 10.1038/s41467-019-10115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhong H., Liu G., Jiang Y., Yang J., Liu Y., Yang X., Liu Z., Zeng G. Transport of bacteria in porous media and its enhancement by surfactants for bioaugmentation: A review. Biotechnol. Adv. 2017;35:490–504. doi: 10.1016/j.biotechadv.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Munir M.T., Pailhoriès H., Aviat F., Lepelletier D., Pape P.L., Dubreil L., Irle M., Buchner J., Eveillard M., Federighi M., et al. Hygienic perspectives of wood in healthcare buildings. Hygiene. 2021;1:12–23. doi: 10.3390/hygiene1010002. [DOI] [Google Scholar]
- 5.Siedenbiedel F., Tiller J.C. Antimicrobial polymers in solution and on surfaces: Overview and functional principles. Polymers. 2012;4:46–71. doi: 10.3390/polym4010046. [DOI] [Google Scholar]
- 6.Kenawy E.R., Worley S.D., Broughton R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules. 2007;8:1359–1384. doi: 10.1021/bm061150q. [DOI] [PubMed] [Google Scholar]
- 7.Muñoz-Bonilla A., Fernández-García M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012;37:281–339. doi: 10.1016/j.progpolymsci.2011.08.005. [DOI] [Google Scholar]
- 8.Divya K., Vijayan S., George T.K., Jisha M.S. Antimicrobial properties of chitosan nanoparticles: Mode of action and factors affecting activity. Fibers Polym. 2017;18:221–230. doi: 10.1007/s12221-017-6690-1. [DOI] [Google Scholar]
- 9.Chen J., Dong Y., Xiao C., Tao Y., Wang X. Organocatalyzed ring-opening polymerization of cyclic lysine derivative: Sustainable access to cationic poly(ϵ-lysine) Mimics. Macromolecules. 2021;54:2226–2231. doi: 10.1021/acs.macromol.0c02689. [DOI] [Google Scholar]
- 10.Charnley M., Textor M., Acikgoz C. Designed polymer structures with antifouling–antimicrobial properties. React. Funct. Polym. 2011;71:329–334. doi: 10.1016/j.reactfunctpolym.2010.10.013. [DOI] [Google Scholar]
- 11.Noimark S., Weiner J., Noor N., Allan E., Williams C.K., Shaffer M.S.P., Parkin I.P. Dual-mechanism antimicrobial polymer-ZnO nanoparticle and crystal violet-encapsulated silicone. Adv. Funct. Mater. 2015;25:1367–1373. doi: 10.1002/adfm.201402980. [DOI] [Google Scholar]
- 12.Li X., Liu Y., Jiang Z., Li R., Ren X., Huang T.S. Synthesis of an N-halamine monomer and its application in antimicrobial cellulose via an electron beam irradiation process. Cellulose. 2015;22:3609–3617. doi: 10.1007/s10570-015-0763-3. [DOI] [Google Scholar]
- 13.Álvarez-Paino M., Muñoz-Bonilla A., Fernández-García M. Antimicrobial polymers in the nano-world. Nanomaterials. 2017;7:48. doi: 10.3390/nano7020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vilas J.L., Laza J.M., Garay M.T., Rodríguez M., León L.M. Unsaturated polyester resins cure: Kinetic, rheologic, and mechanical-dynamical analysis. I. Cure kinetics by DSC and TSR. J. Appl. Polym. Sci. 2001;79:447–457. doi: 10.1002/1097-4628(20010118)79:3<447::AID-APP70>3.0.CO;2-M. [DOI] [Google Scholar]
- 15.Ribeiro F.V., Casati M.Z., Casarin R.C., Corrêa M.G., Cirano F.R., Negri B.M., Pimentel S.P. Impact of a triclosan-containing toothpaste during the progression of experimental peri-implant mucositis: Clinical parameters and local pattern of osteo-immunoinflammatory mediators in peri-implant fluid. J. Periodontol. 2018;89:203–212. doi: 10.1002/JPER.17-0302. [DOI] [PubMed] [Google Scholar]
- 16.Halla N., Fernandes I.P., Heleno S.A., Costa P., Boucherit-Otmani Z., Boucherit K., Rodrigues A.E., Ferreira I.C.F.R., Barreiro M.F. Cosmetics preservation: A review on present strategies. Molecules. 2018;23:1571. doi: 10.3390/molecules23071571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher L.E., Hook A.L., Ashraf W., Yousef A., Barrett D.A., Scurr D.J., Chen X., Smith E.F., Fay M., Parmenter C.D.J., et al. Biomaterial modification of urinary catheters with antimicrobials to give long-term broadspectrum antibiofilm activity. J. Control. Release. 2015;202:57–64. doi: 10.1016/j.jconrel.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 18.Kampf G., Kramer A. Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clin. Microbiol. Rev. 2004;17:863–893. doi: 10.1128/CMR.17.4.863-893.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wegehaupt F.J., Tauböck T.T., Attin T., Belibasakis G.N. Influence of light-curing mode on the cytotoxicity of resin-based surface sealants. BMC Oral Health. 2014;14:48. doi: 10.1186/1472-6831-14-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunha D.A., Rodrigues N.S., Souza L.C., Lomonaco D., Rodrigues F.P., Degrazia F.W., Collares F.M., Sauro S., de Paulo Aragão Saboia V. Physicochemical and microbiological assessment of an experimental composite doped with Triclosan-loaded halloysite nanotubes. Materials. 2018;11:1080. doi: 10.3390/ma11071080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Degrazia F.W., Genari B., Leitune V.C.B., Arthur R.A., Luxan S.A., Samuel S.M.W., Collares F.M., Sauro S. Polymerisation, antibacterial and bioactivity properties of experimental orthodontic adhesives containing triclosan-loaded halloysite nanotubes. J. Dent. 2018;69:77–82. doi: 10.1016/j.jdent.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Genari B., Leitune V.C.B., Jornada D.S., Camassola M., Arthur R.A., Pohlmann A.R., Guterres S.S., Collares F.M., Samuel S.M.W. Antimicrobial effect and physicochemical properties of an adhesive system containing nanocapsules. Dent. Mater. 2017;33:735–742. doi: 10.1016/j.dental.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Machado A.H.S., Garcia I.M., Motta A.d.S.d., Leitune V.C.B., Collares F.M. Triclosan-loaded chitosan as antibacterial agent for adhesive resin. J. Dent. 2019;83:33–39. doi: 10.1016/j.jdent.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Kamalipour J., Masoomi M., Khonakdar H.A., Razavi S.M.R. Preparation and release study of Triclosan in polyethylene/Triclosan anti-bacterial blend. Colloids Surf. B Biointerfaces. 2016;145:891–898. doi: 10.1016/j.colsurfb.2016.05.093. [DOI] [PubMed] [Google Scholar]
- 25.Kaffashi B., Davoodi S., Oliaei E. Poly(ϵ-caprolactone)/triclosan loaded polylactic acid nanoparticles composite: A long-term antibacterial bionanocomposite with sustained release. Int. J. Pharm. 2016;508:10–21. doi: 10.1016/j.ijpharm.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Palza H., Quijada R., Delgado K. Antimicrobial polymer composites with copper micro- and nanoparticles: Effect of particle size and polymer matrix. J. Bioact. Compat. Polym. 2015;30:366–380. doi: 10.1177/0883911515578870. [DOI] [Google Scholar]
- 27.Yılmaz Atay H., Çelik E. Investigations of antibacterial activity of chitosan in the polymeric composite coatings. Prog. Org. Coat. 2017;102:194–200. doi: 10.1016/j.porgcoat.2016.10.013. [DOI] [Google Scholar]
- 28.Shafiq M., Yasin T., Shaista R.A.M. Structural, thermal, and antibacterial properties of chitosan/ZnO composites. Polym. Polym. Compos. 2013;16:101–113. doi: 10.1002/pc.22636. [DOI] [Google Scholar]
- 29.Yudovin-Farber I., Beyth N., Weiss E.I., Domb A.J. Antibacterial effect of composite resins containing quaternary ammonium polyethyleneimine nanoparticles. J. Nanopart. Res. 2010;12:591–603. doi: 10.1007/s11051-009-9628-8. [DOI] [Google Scholar]
- 30.Ferfera-Harrar H., Aiouaz N., Dairi N., Hadj-Hamou A.S. Preparation of chitosan-g-poly(acrylamide)/montmorillonite superabsorbent polymer composites: Studies on swelling, thermal, and antibacterial properties. J. Appl. Polym. Sci. 2014;131:39747. doi: 10.1002/app.39747. [DOI] [Google Scholar]
- 31.Zapata P.A., Larrea M., Tamayo L., Rabagliati F.M., Azócar M.I., Páez M. Polyethylene/silver-nanofiber composites: A material for antibacterial films. Mater. Sci. Eng. C. 2016;69:1282–1289. doi: 10.1016/j.msec.2016.08.039. [DOI] [PubMed] [Google Scholar]
- 32.Weng Y., Howard L., Guo X., Chong V.J., Gregory R.L., Xie D. A novel antibacterial resin composite for improved dental restoratives. J. Mater. Sci. Mater. Med. 2012;23:1553–1561. doi: 10.1007/s10856-012-4629-z. [DOI] [PubMed] [Google Scholar]
- 33.Rogalsky S., Bardeau J.F., Wu H., Lyoshina L., Bulko O., Tarasyuk O., Makhno S., Cherniavska T., Kyselov Y., Koo J.H. Structural, thermal and antibacterial properties of polyamide 11/polymeric biocide polyhexamethylene guanidine dodecylbenzenesulfonate composites. J. Mater. Sci. 2016;51:7716–7730. doi: 10.1007/s10853-016-0054-x. [DOI] [Google Scholar]
- 34.Woo G.L.Y., Mittelman M.W., Santerre J.P. Synthesis and characterization of a novel biodegradable antimicrobial polymer. Biomaterials. 2000;21:1235–1246. doi: 10.1016/S0142-9612(00)00003-X. [DOI] [PubMed] [Google Scholar]
- 35.Zhao J., Ma L., Millians W., Wu T., Ming W. Dual-functional antifogging/antimicrobial polymer coating. ACS Appl. Mater. Interfaces. 2016;8:8737–8742. doi: 10.1021/acsami.6b00748. [DOI] [PubMed] [Google Scholar]
- 36.Jiang J., Zhu L., Zhu L., Zhang H., Zhu B., Xu Y. Antifouling and antimicrobial polymer membranes based on bioinspired polydopamine and strong hydrogen-bonded poly(n-vinyl pyrrolidone) ACS Appl. Mater. Interfaces. 2013;5:12895–12904. doi: 10.1021/am403405c. [DOI] [PubMed] [Google Scholar]
- 37.Namivandi-Zangeneh R., Sadrearhami Z., Bagheri A., Sauvage-Nguyen M., Ho K.K.K., Kumar N., Wong E.H.H., Boyer C. Nitric oxide-loaded antimicrobial polymer for the synergistic eradication of bacterial biofilm. ACS Macro Lett. 2018;7:592–597. doi: 10.1021/acsmacrolett.8b00190. [DOI] [PubMed] [Google Scholar]
- 38.Piotto S., Concilio S., Sessa L., Iannelli P., Porta A., Calabrese E.C., Galdi M.R., Incarnato L. Novel antimicrobial polymer films active against bacteria and fungi. Polym. Compos. 2013;34:1489–1492. doi: 10.1002/pc.22410. [DOI] [Google Scholar]
- 39.Zakiruly O. Polymer composite for production of multifunctional material. Kazakhstan Pat. 2019;33515:1–3. [Google Scholar]
- 40.Djouonkep L.D.W., Tamo A.K., Doench I., Selabi N.B.S., Ilunga E.M., Lenwoue A.R.K., Gauthier M., Cheng Z., Osorio-Madrazo A. Synthesis of high performance thiophene–aromatic polyesters from bio-sourced organic acids and polysaccharide-derived diol: Characterization and degradability studies. Molecules. 2022;27:325. doi: 10.3390/molecules27010325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiegand C., Völpel A., Ewald A., Remesch M., Kuever J., Bauer J., Griesheim S., Hauser C., Thielmann J., Tonndorf-Martini S., et al. Critical physiological factors influencing the outcome of antimicrobial testing according to ISO 22196/JIS Z 2801. PLoS ONE. 2018;13:e0194339. doi: 10.1371/journal.pone.0194339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bojar W., Kazmierska K., Szalwinski M., Zareba T. Triclosan-coated sutures in oral surgery. Adv. Clin. Exp. Med. 2009;18:401–405. [Google Scholar]
- 43.Jones G.L., Muller C.T., O’Reilly M., Stickler D.J. Effect of triclosan on the development of bacterial biofilms by urinary tract pathogens on urinary catheters. J. Antimicrob. Chemother. 2006;57:266–272. doi: 10.1093/jac/dki447. [DOI] [PubMed] [Google Scholar]
- 44.Zu G., Steinmüller M., Keskin D., Van Der Mei H.C., Mergel O., Van Rijn P. Antimicrobial nanogels with nanoinjection capabilities for delivery of the hydrophobic antibacterial agent Triclosan. ACS Appl. Polym. Mater. 2020;2:5779–5789. doi: 10.1021/acsapm.0c01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maiden M.M., Hunt A.M.A., Zachos M.P., Gibson J.A., Hurwitz M.E., Mulks M.H., Waters C.M. Triclosan is an aminoglycoside adjuvant for eradication of Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2018;62:e00146-18. doi: 10.1128/AAC.00146-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raut J., Chauhan N., Shinde R., Karuppayil S.M. Inhibition of planktonic and biofilm growth of Candida albicans reveals novel antifungal activity of caffeine. J. Med. Plant. Res. 2013;7:777. doi: 10.5897/JMPR12.765. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in Supplementary Material.