Figure 2.
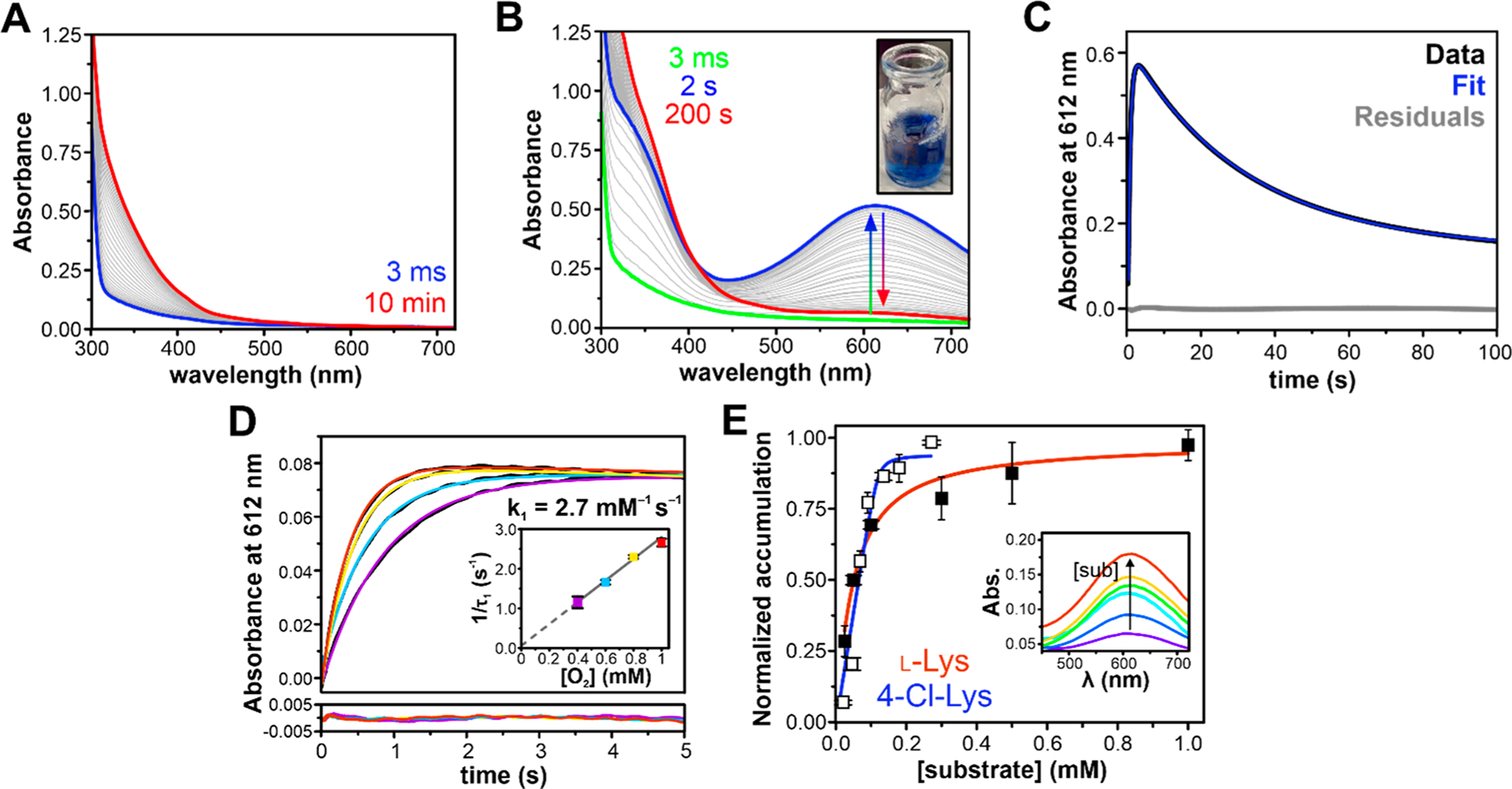
Substrate-triggered formation of an oxygenated intermediate in BesC. (A) Rapid mixing of diferrous BesC (300 μM postmix concentration) with O2-saturated buffer (1 mM postmix) in the absence of substrate results in slow autoxidation. (B) Substrate-bound BesC (300 μM BesC, 1.5 mM 4-Cl-Lys postmix) activates oxygen to produce a blue 612 nm chromophore, shown at a 1 mM O2 postmix concentration in the inset. (C) The single-wavelength time-course measured at 612 nm can be fit with a two-summed exponential expression showing single formation and decay phases. (D) The formation rate of BesC-P is linearly dependent on [O2], measured at a postmix O2 concentrations of 1.0 mM (red), 0.8 mM (yellow), 0.6 mM (blue), and 0.4 mM (purple) and a BesC concentration of 50 μM. (E) Accumulation of BesC-P shows a hyperbolic dependence on substrate concentration and is used to determine the relative efficiencies for l-(Cl)-Lys binding and O2 activation. Postmixing concentration of protein was 100 μM, and substrate concentrations used were 25, 50, 100, 300, 500, and 1000 μM for Lys (filled squares) and 25, 50, 75, 100, 125, 175, and 250 μM for 4-Cl-Lys (open squares). Inset: Representative PDA spectra of maximum BesC-P accumulation at different concentrations of 4-Cl-Lys, with warmer colors indicating higher concentrations. All experiments were performed at 4 °C.
