Figure 7.
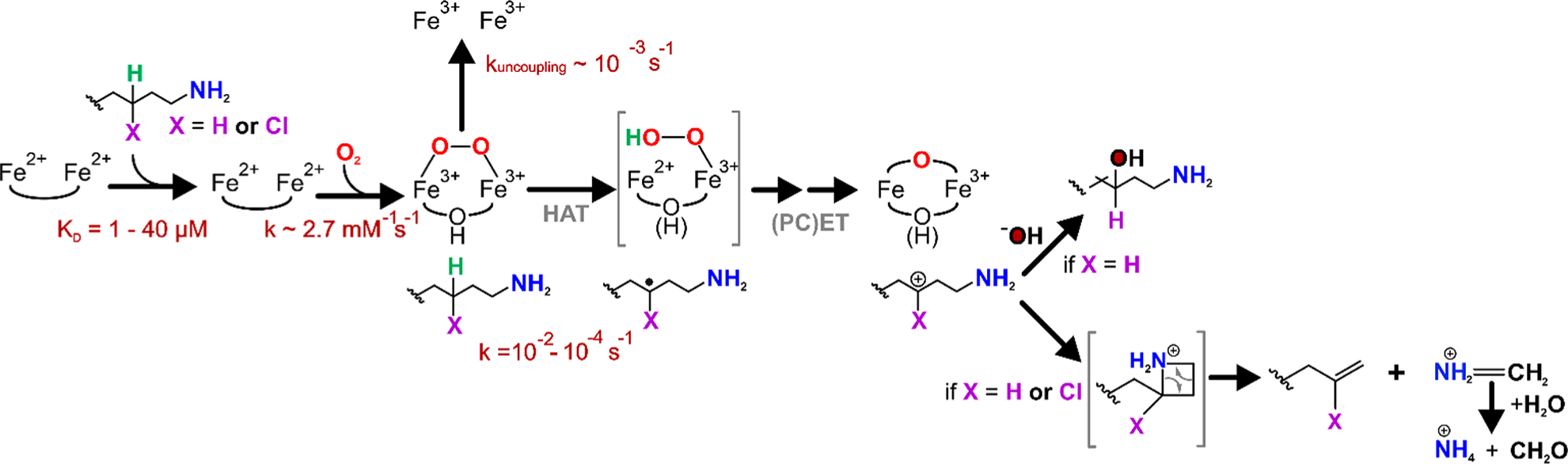
Proposed mechanism for oxidative C–C cleavage by BesC. Substrate-triggered activation of O2 yields the diferric-peroxo intermediate BesC-P, which branches between C4–H cleavage and uncoupling depending on the identity of X (H or Cl). HAT by BesC-P may be followed by an iron(II/III)-hydroperoxo species and subsequent iron(III/IV)-oxo/hydroxo species (bracketed because these have not been directly observed). (PC)ET then yields the carbocation intermediate. At this point, the reaction may proceed through a cyclic intermediate before cleaving to yield (4-chloro)-l-allylglycine and methylene imine. The latter may be nonenzymatically hydrolyzed to ammonium and formaldehyde. An alternative fate of the carbocation intermediate is quenching by a solvent-derived hydroxide, observed when X = H.
