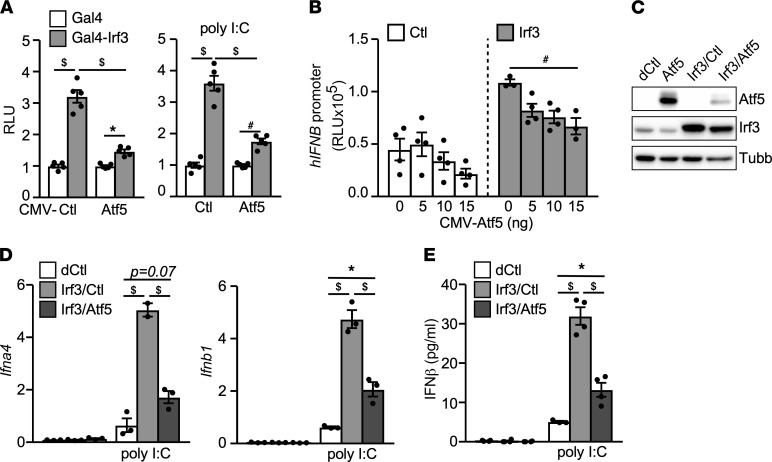
Figure 5. Atf5 suppresses IFN-I response through Irf3.
(A) Atf5 inhibits the transactivation activity of Irf3. Hepa1–6 cells were cotransfected with a luciferase reporter driven by SV40 promoter with 4 copies of the Gal4-binding site and an expression vector for Gal4 or Gal4-Irf3, together with a CMV control (Ctl) or CMV-Atf5 expression vector. CMV-β-galactosidase was included to monitor the transfection efficiency. Cells were transfected with/without 100 ng/well Poly I:C for the last 16 hours. The luciferase activity was normalized by the β-galactosidase activity. RLU was presented as fold change of Gal4-Irf3 versus Gal4. n = 5. (B) Control or Irf3 overexpressing Hepa1–6 stable cell lines were cotransfected with human IFNβ promotor reporter, CMV-β-galactosidase, and either the CMV control or increasing amounts of CMV-Atf5 expression vector (total amount of plasmid DNA was kept same with the control vector). The luciferase activity was normalized by the β-galactosidase activity to determine the RLU. n = 5. (C) Immunoblotting showing the protein level of Atf5 (probed with anti-HA antibody) and Irf3 in Hepa1–6 “dual” stable lines expressing control empty vector (dCtl), Irf3 (Irf3/Ctl), or Irf3 together with Atf5 (Irf3/Atf5). Anti-Irf3 antibody detected both endogenous and overexpressed Irf3 protein. Tubb protein level was loading control. The Atf5 expressing Hepa1–6 “single” stable line (Supplemental Figure 4B) was included for comparison. (D) Ifna4 and Ifnb1 gene expression and (E) IFN-β protein secretion in control, Irf3, and Irf3/Atf5 overexpressing Hepa1–6 stable lines stimulated with/without 100 ng/well Poly I:C for 16 hours. n = 3. Experiments repeated 3 times. Values are presented as mean ± SEM. Significance was determined by 1-way ANOVA followed by Holm-Šidák multiple comparisons test. *P < 0.05; #P < 0.01; $P < 0.001.